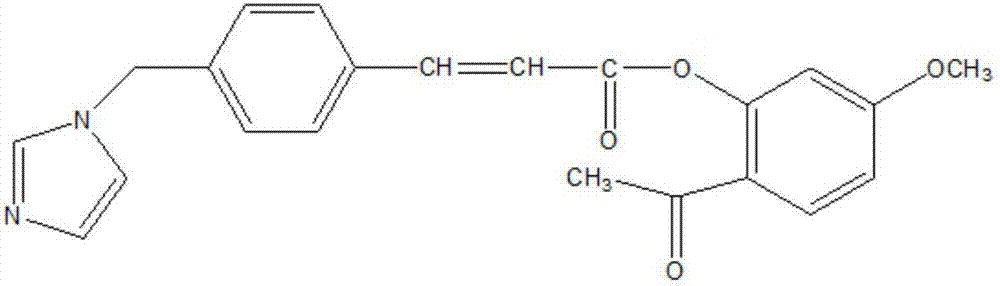
Method for preparing paeonol-ozagrel conjugate lipidosome through ethanol injection method
A technology of paeonol and conjugates, which is applied in the field of pharmaceutical preparation, can solve the problems of limitations, poor water solubility and fat solubility, etc., and achieve the effects of easy mastery, high drug loading and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Measure 20 mL of phosphate buffer solution into a stoppered Erlenmeyer flask, put in a magnet, and stir on a collector heating stirrer with a rotation speed of 350 r / min and a set temperature of 35 °C. Precisely weigh 6 mg of ozagrel, 100 mg of soybean lecithin, and 5 mg of cholesterol into a stoppered Erlenmeyer flask, add 3 mL of absolute ethanol and sonicate for 4 minutes to dissolve them, inject the above drugs into the phosphate buffer slowly and uniformly with a 1 mL syringe, and continue After stirring for 2 hours, the final suspension was passed through a 0.80 μm microporous membrane to size the particles to obtain paeonol-ozagrel conjugate liposomes.
[0025] The drug loading and encapsulation efficiency of the paeonol-ozagrel conjugate liposome were determined to be 9.72% and 86.3%, respectively.
Embodiment 2
[0027] Measure 20 mL of phosphate buffer solution into a stoppered Erlenmeyer flask, put it into a magnet, and stir on a collector heating stirrer with a rotation speed of 300 r / min and a set temperature of 40 °C. Precisely weigh 9 mg of ozagrel, 150 mg of soybean lecithin, and 7.5 mg of cholesterol into a stoppered Erlenmeyer flask, add 3 mL of absolute ethanol and sonicate for 4 minutes to dissolve them, and inject the above drugs into the phosphate buffer slowly and uniformly with a 1 mL syringe. Stirring was continued for 2 hours, and the finally obtained suspension was sized through a 0.80 μm microporous membrane to obtain paeonol-ozagrel conjugate liposomes. The drug loading and encapsulation efficiency of the paeonol-ozagrel conjugate liposome were determined to be 9.92% and 87.4%, respectively.
Embodiment 3
[0029] Measure 20 mL of phosphate buffer solution into a stoppered Erlenmeyer flask, put it into a magnet, and stir on a collector heating stirrer with a rotation speed of 300 r / min and a set temperature of 40 °C. Precisely weigh 6 mg of ozagrel, 100 mg of soybean lecithin, and 10 mg of cholesterol into a stoppered Erlenmeyer flask, add 3 mL of absolute ethanol and sonicate for 4 minutes to dissolve them, inject the above drugs into the phosphate buffer slowly and uniformly with a 1 mL syringe, and continue After stirring for 2 hours, the final suspension was passed through a 0.80 μm microporous membrane to size the particles to obtain paeonol-ozagrel conjugate liposomes. The drug loading and encapsulation efficiency of paeonol-ozagrel conjugate liposomes were determined to be 9.62% and 84.6%, respectively.
[0030] Preparation Method 2 - Reverse Evaporation Method
[0031] Ratio of each component of paeonol-ozagrel conjugate liposome: paeonol-ozagrel conjugate: phospholipid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com