Injection ozagrel sodium freeze-dried powder for treating cerebral infarction
A technology for ozagrel sodium and injection, which is applied in the field of medicine, can solve problems such as unstable quality of ozagrel sodium freeze-dried powder for injection, non-standard step control process, increased risk of clinical use, etc., to achieve suitable The effect of large production requirements, stable and controllable quality, and fewer types of auxiliary materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1: Preparation of sodium ozagrel crystals
[0014] (1) Grind the crude sodium ozagrel, pass through a 90-mesh sieve, add it to 45% N-methylacetamide aqueous solution, and heat up to 30°C while stirring; mix the crude sodium ozagrel with 45% N- The weight ratio of methyl acetamide aqueous solution is 1:10; the stirring speed is 180 rpm; add activated carbon, stir for 50 minutes and then sterile filter; (2) add ether while stirring, and lower the temperature to -15°C at the same time; the stirring speed Be 240 revs / min; The weight of ether is 7 times of the mixed solution weight of ozagrel sodium, 45% N-methylacetamide aqueous solution weight, and adding speed is 95 milliliters / minute; Cooling speed is 10 ℃ / hour; (3 ) After adding the mixed solvent, the obtained crystals were left to stand for crystallization; filtered, washed, and vacuum-dried for 6 hours to obtain the sodium ozagrel compound.
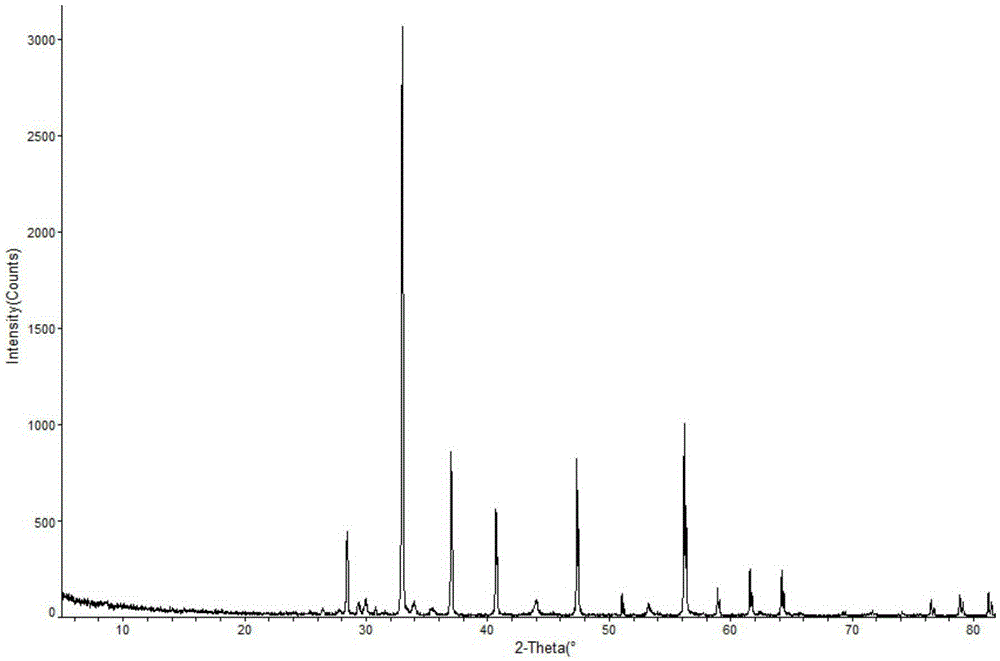
[0015] The prepared sodium ozagrel crystal is measured by powder...
Embodiment 2
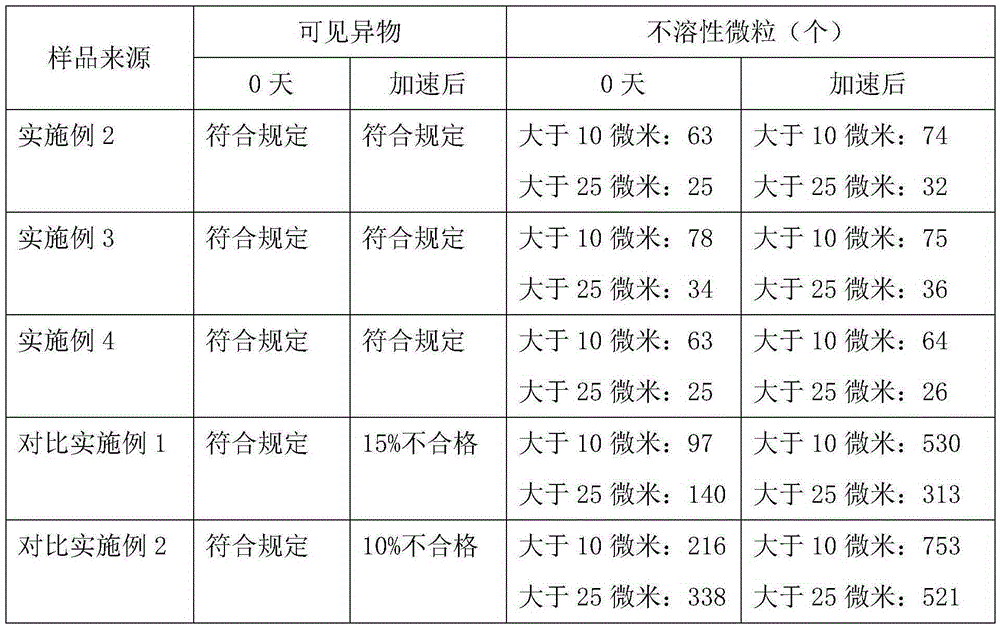
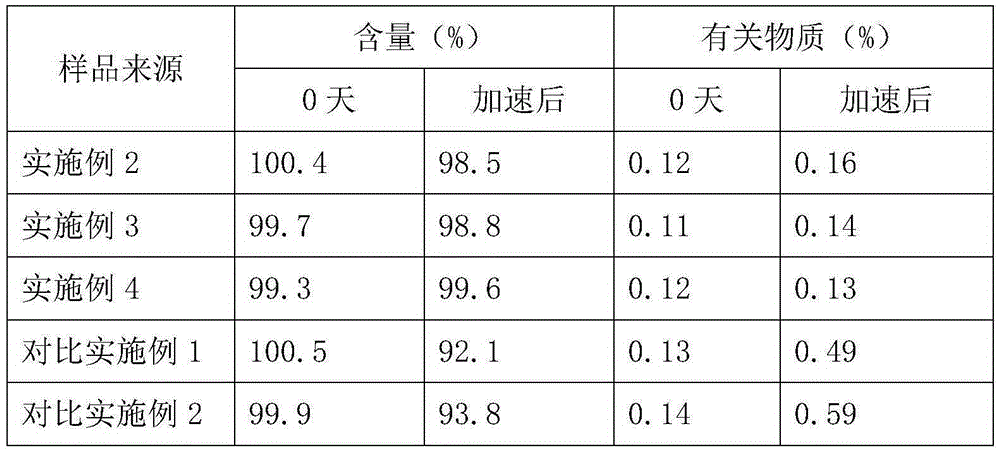
[0016]Embodiment 2: get ozagrel sodium 30g, mannitol 60g, meglumine 10g, water for injection and add to 6000ml, make 1000 bottles altogether, its preparation process is: take prescription quantity ozagrel sodium and put in pressure vessel, add The water for injection with a total volume of 80%, under the condition that the relative pressure is 0.06MPa, heat and adjust the water temperature to 105°C, and stir for 0.5 hour to obtain a sodium ozagrel solution, then add 2% of the total mass of the solution and stir for 2.5 hours. Filtrate with a 0.45 μm microporous membrane under heat preservation conditions, add 5% sodium hydroxide solution to the obtained filtrate to adjust the pH value to 11.5 under the condition of heat preservation at 85 ° C, and lower it to room temperature, add the prescribed amount of mannitol and meglumine, and use Dilute to the full volume with water for injection, filter it with a 0.22 μm microporous membrane under aseptic conditions, fill it in a vial, ...
Embodiment 3
[0017] Embodiment 3: get ozagrel sodium 30g, mannitol 70g, meglumine 15g, water for injection and add to 6000ml, make 1000 bottles altogether, its preparation process is: take prescription quantity ozagrel sodium and put in pressure vessel, add The water for injection with a total volume of 80%, under the condition that the relative pressure is 0.07MPa, heat and adjust the water temperature to 115°C, and stir for 0.5 hour to obtain a sodium ozagrel solution, then add activated carbon with 2% of the total mass of the solution and stir for 2.5 hours. Filtrate with a 0.45 μm microporous membrane under heat preservation conditions, add 5% sodium hydroxide solution to the obtained filtrate to adjust the pH value to 11.5 under the condition of heat preservation at 85 ° C, and lower it to room temperature, add the prescribed amount of mannitol and meglumine, and use Dilute to the full volume with water for injection, filter it with a 0.22 μm microporous membrane under aseptic conditio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com