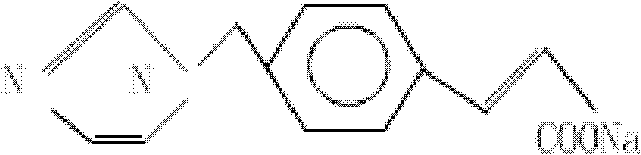
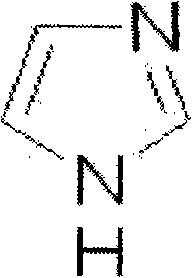Patents
Literature
31 results about "Sodium ozagrel" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
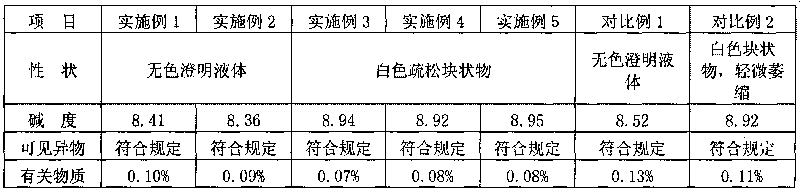
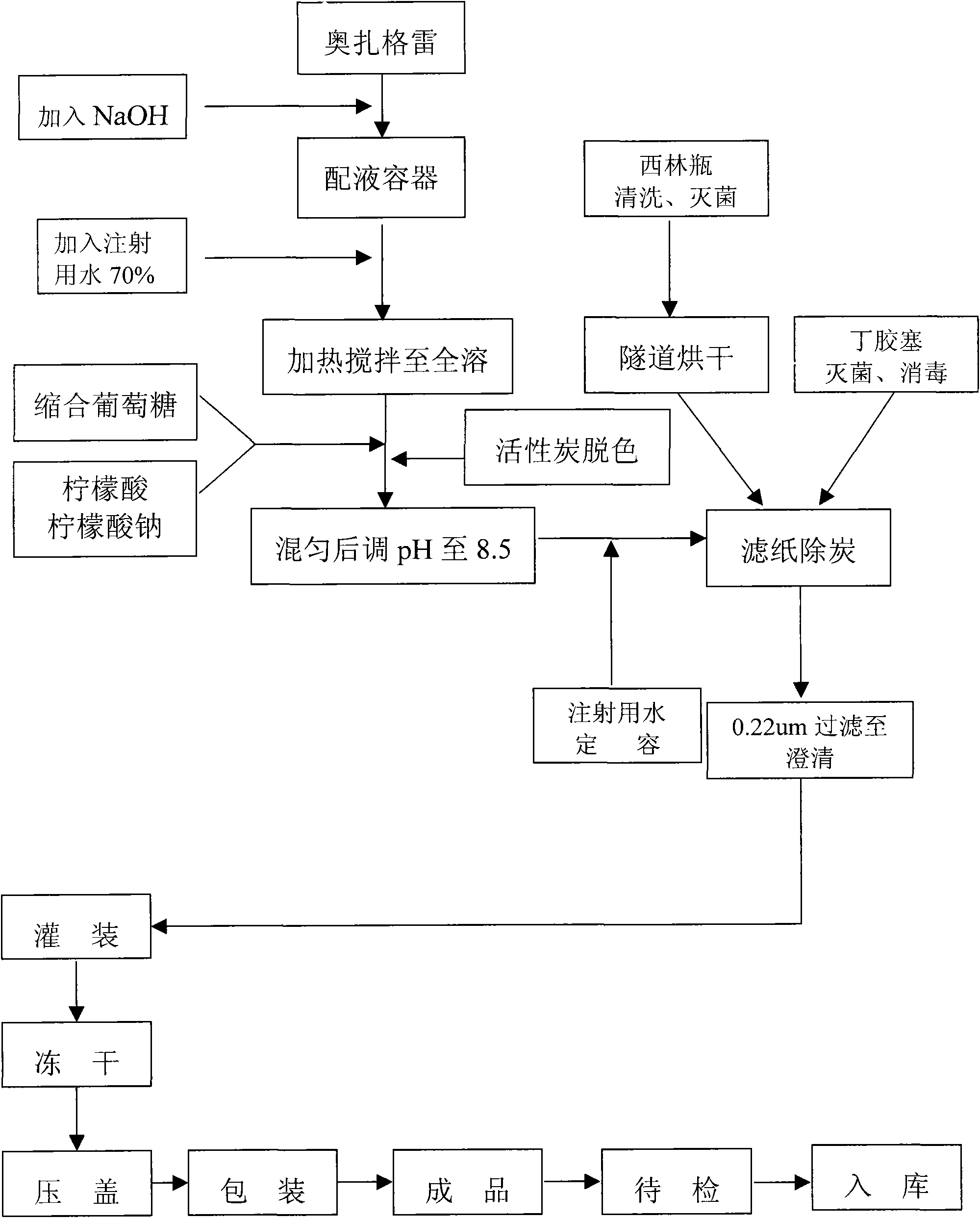
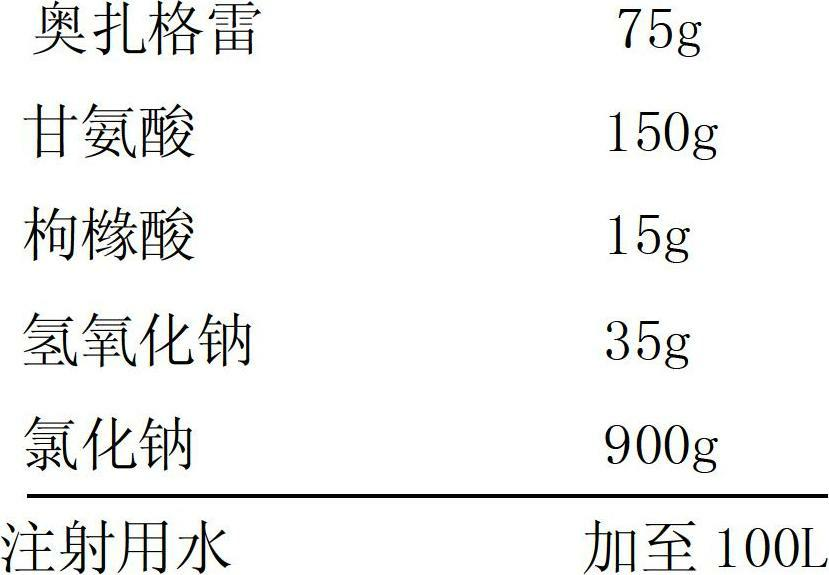
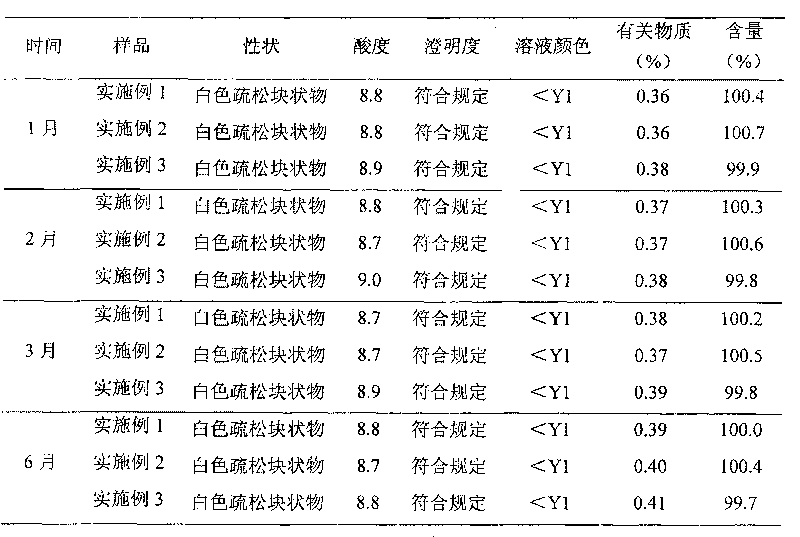
Ozagrel sodium injection and preparation method thereof
ActiveCN101695475AQuality assuranceEnsure stabilityOrganic active ingredientsPowder deliverySolventExcipient
The invention relates to an ozagrel sodium injection and a preparation method thereof, in particular to an ozagrel sodium injection for treating acute thrombotic cerebral infarction and movement disorders concomitant with cerebral infarction, preferably injection solution and lyophilized powder injection. The ozagrel sodium injection mainly comprises ozagrel serving as an active component and sodium hydroxide and citric acid serving as auxiliary materials. Solvent in the injection solution is water for injection. Excipient in the lyophilized powder injection is mannitol and / or sorbitol which can be combined in any one or two optional medicinal proportions, or no excipient is added.
Owner:HAINAN LEVTEC PHARMA
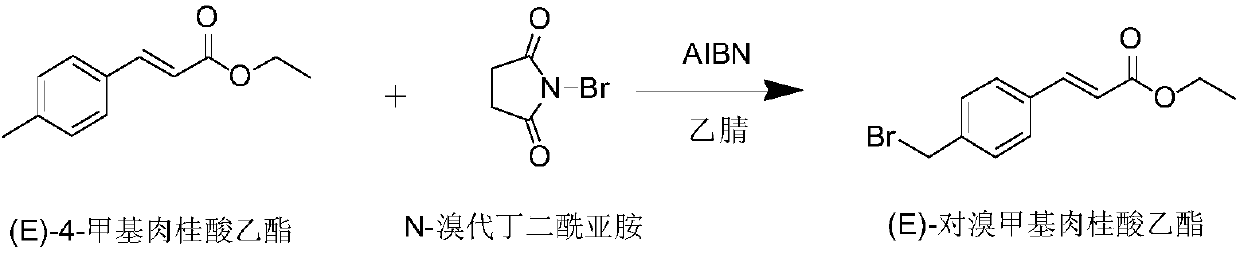
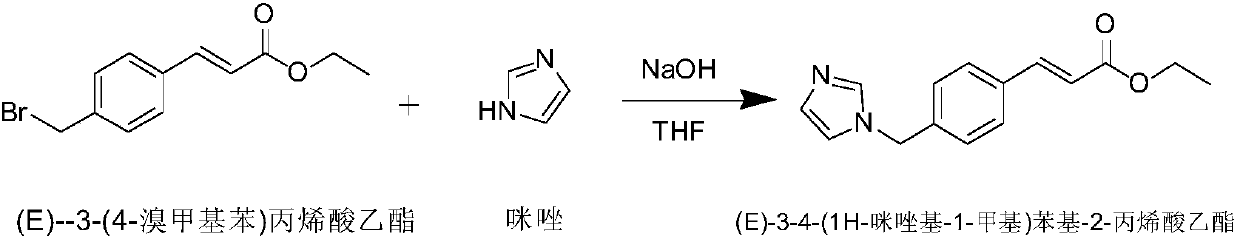
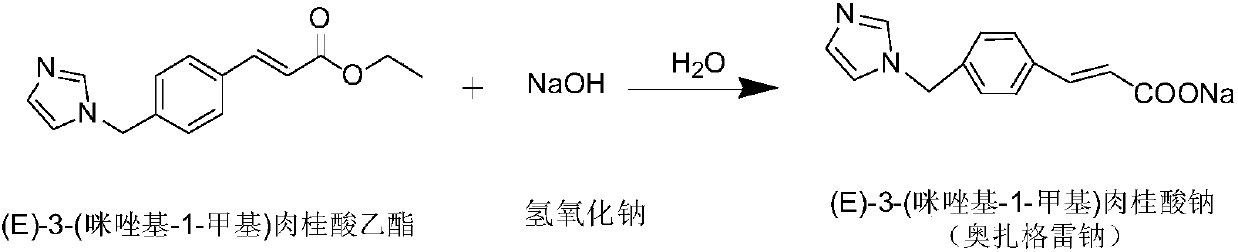
Preparation method of Ozagrel sodium
The invention discloses a preparation method of Ozagrel sodium. The method comprises the following steps: performing a bromination reaction on ethyl 4-methylcinnamate and N-bromo-succinimide by takingacetonitrile as a solvent under the triggering of azodiisobutyronitrile, so as to obtain ethyl 4-bromomethylcinnamate; performing a condensation cyclization reaction on the ethyl 4-bromomethylcinnamate and imidazole by taking sodium hydroxide as an acid-binding agent and taking tetrahydrofuran as a solvent, so as to obtain imidazole ethyl 4-methylcinnamate; performing alkali hydrolysis on the imidazole ethyl 4-methylcinnamate, so as to obtain the Ozagrel sodium. According to the preparation method, the condensation cyclization reaction is performed by taking the sodium hydroxide as the acid-binding agent and taking the tetrahydrofuran as the solvent, so that the finally obtained product does not contain toxic components, the yield and the purity of the product can be effectively improved,and the content of genotoxic impurities (the ethyl 4-bromomethylcinnamate and ethyl 4-dibromomethylcinnamate) in the product is zero.
Owner:浙江科瑞医药科技有限公司
A more stable sodium ozagrel compound and pharmaceutical composition thereof
InactiveCN102276532AEasy to manufacturePreparation belongs to process improvementPowder deliveryOrganic active ingredientsSolventEthyl acetate
The present invention relates to a more stable sodium ozagrel compound and a pharmaceutical composition, which comprises recrystallizing the crude product sodium ozagrel with ethyl acetate:methanol=1:1 as a solvent for 1-3 times, and simultaneously using activated carbon White crystals are obtained after decolorization.
Owner:贺金凤
Thrombosis resisting composition
InactiveCN1436571AGood effectImprove solubilityAnthropod material medical ingredientsPeptide/protein ingredientsDiseaseSide effect
The thrombosis resisting composition consists of thromboxane A2(TXA2) synthetase inhibitor, thrombolytic medicine and / or thrombocyte resisting medicine; and can dissolving thrombus. The composition is obviously superior to single thromboxane A2(TXA2) synthetase inhibitor, and has high effect of preventing and treating thrombus diseases, small dosage and less side effect. Both extracorporeal and intracorporeal experiments shows that the composition has excellent direct thrombus dissolving effect and thrombocyte aggregation inhibiting effect. When the composition of sodium Ozagrel is used in treating acute cerebral thrombus, the clinical total effective rate is 100% and side effect originating rate is 2.1%. While only sodium Ozagrel is used, the corresponding data is 49.2% and 7.6% separately.
Owner:田兆军
Ozagrel sodium drug combination for injection
InactiveCN102846561AFix stability issuesHigh yieldPowder deliveryOrganic active ingredientsProduction rateMANNITOL/SORBITOL
The invention discloses an ozagrel sodium drug combination for injection. The ozagrel sodium injection solution consists of ozagrel sodium, mannitol and anhydrous sodium carbonate, wherein each piece contains 20-80 mg of ozagrel sodium, 20-80 mg of mannitol and 5-15 mg of anhydrous sodium carbonate. The drug combination is prepared by a method comprising the following steps: taking a recipe quantity of injection water, adding the mannitol and the anhydrous sodium carbonate and stirring until the mannitol and the anhydrous sodium carbonate are dissolved; adding the ozagrel sodium, and stirring until the ozagrel sodium is fully dissolved; regulating a pH value between 7.7 and 8.7; adding medicinal carbon and stirring; leaching, replenishing the injection water to a full quantity and uniformly mixing; finely filtering; encapsulating; freeze-drying; inspecting with a light; and warehousing to obtain the ozagrel sodium drug combination. The ozagrel sodium drug combination has good stability. The production rate of the product is increased, the cost is lowered, and industrialization is realized. The drug combination can be better applied in clinic and has more remarkable advantages.
Owner:TIANJIN SONGRUI MEDICAL TECH
Small-size sodium ozagrel freeze-dried powder needle as well as preparation method and production device thereof
InactiveCN103271881AReduce moisture contentImprove stabilityPowder deliveryOrganic active ingredientsPenicillinFreeze-drying
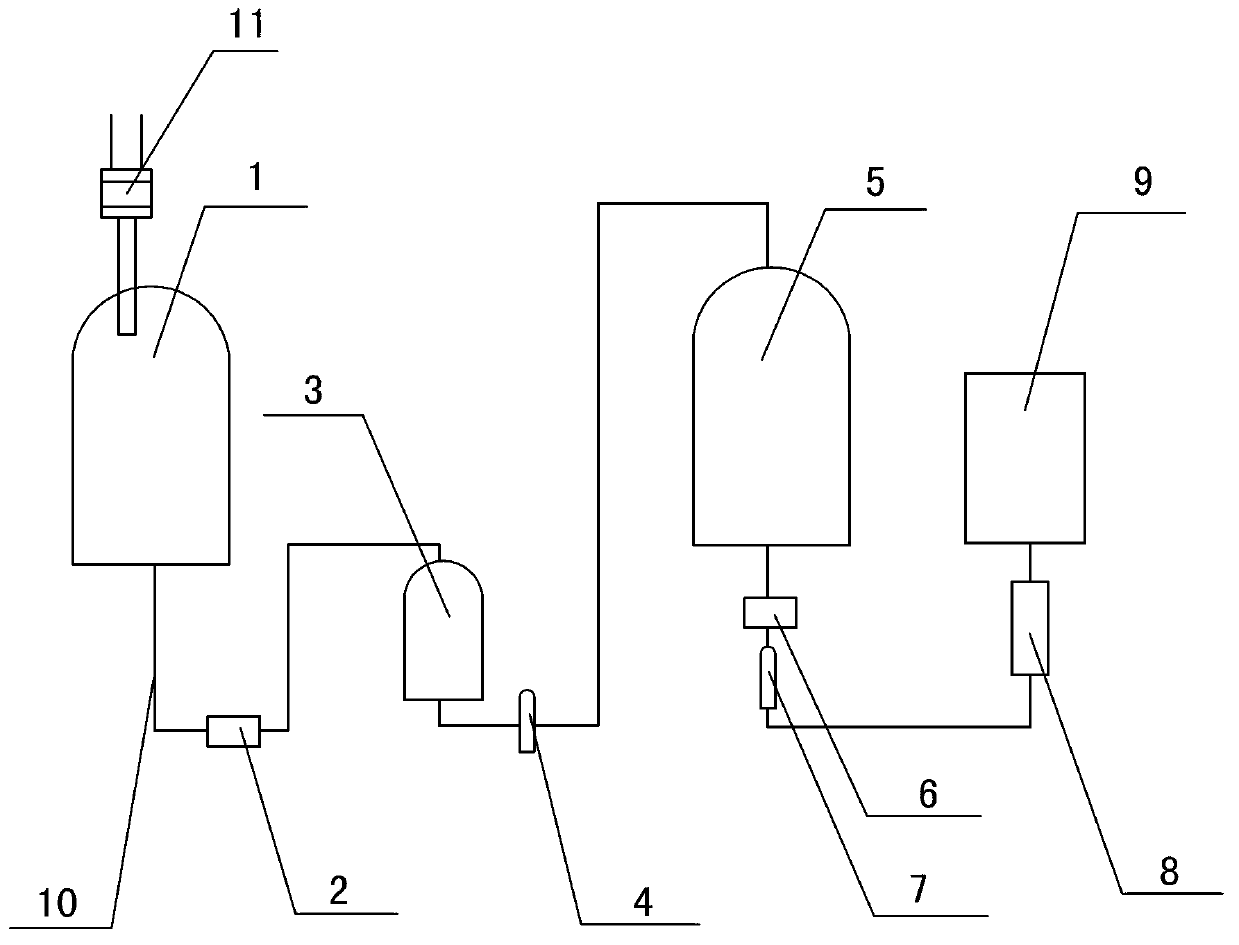
The invention belongs to the technical field of freeze-dried powder needle preparation and relates to a small-size sodium ozagrel freeze-dried powder needle as well as a preparation method and a production device thereof. The small-size sodium ozagrel freeze-dried powder needle is characterized in that sodium ozagrel freeze-dried powder is filled into a 7ml pipe penicillin bottle; and the content of sodium ozagrel in the sodium ozagrel freeze-dried powder needle is 20mg, 40mg or 80mg. The preparation method comprises the following steps of: before preparation, washing the device by a metal complexing agent firstly and then by injection water, and meanwhile, introducing nitrogen; reacting the sodium ozagrel, sodium hydroxide and an auxiliary material; after reaction, adding active carbon; decarbonizing and filtering by a titanium rod, and then carrying out fine filtering; and then filling the powder into the 7ml pipe penicillin bottle, and carrying out freeze-drying, pressing and blocking, as well as cover rolling to obtain the product. According to the small-size sodium ozagrel freeze-dried powder needle as well as the preparation method and the production device thereof disclosed by the invention, a product process is optimized so that the size of the sodium ozagrel freeze-dried powder needle is reduced, the yield of the product is improved, and the packaging and transporting cost is reduced; and as the device is washed by the metal complexing agent, the quality of the product is improved and the medication safety is guaranteed.
Owner:REYOUNG PHARMA
Ozagrel sodium microballoon lyophilized preparation and preparation method thereof
InactiveCN101444491AImprove stabilityNo decompositionPowder deliveryOrganic active ingredientsSodium ozagrelChemistry
The invention relates to an ozagrel sodium microballoon lyophilized preparation and a preparation method thereof. The ozagrel sodium microballoon lyophilized preparation of the invention mainly includes the following components by weight part: 20-80 parts of ozagrel sodium, 50-150 parts of chitosan, 40-100 parts of polyvinyl alcohol, and 50-300 parts of lyophilization protective agent. The ozagrel sodium microballoon lyophilized preparation of the invention has the advantages of good stability and solubility, small grain diameter of lipidosome after hydration, strong targeting property, low production cost and the like.
Owner:HAINAN MEIDA PHARMA
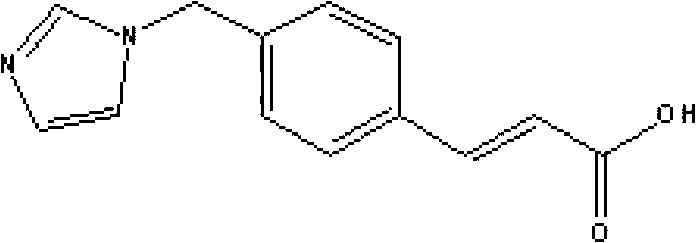
Sodium ozagrel compound, preparation method and drug composition thereof
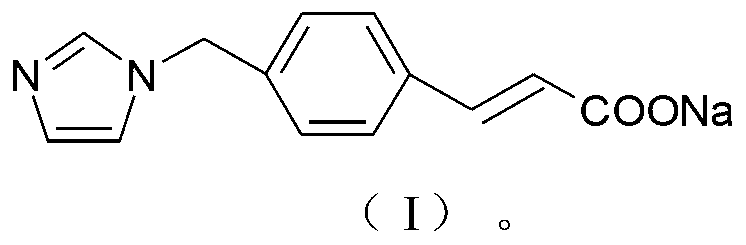
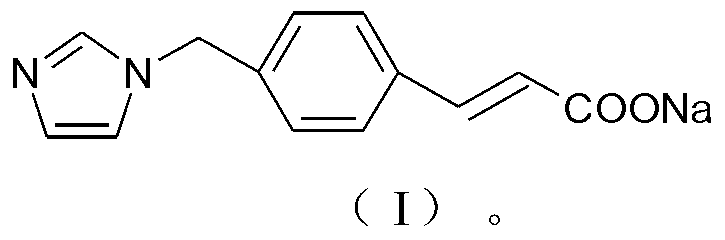
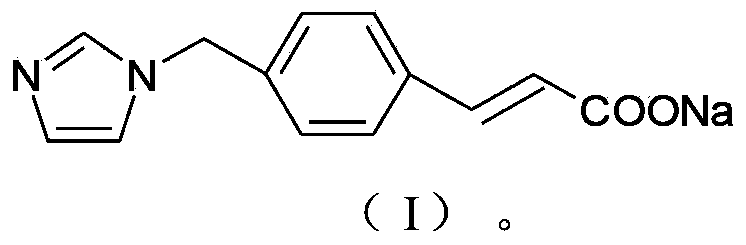
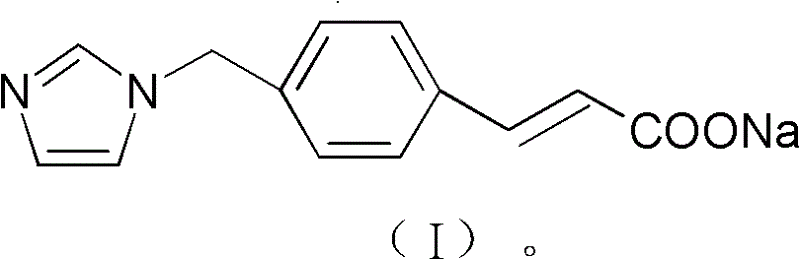
ActiveCN103232395AImprove stabilitySuitable for clinical applicationOrganic active ingredientsOrganic chemistryFreeze-dryingStructural formula
The invention relates to a sodium ozagrel compound and a preparation method thereof. The structural formula of the sodium ozagrel compound is shown in the formula I in the specification. The invention also relates to a drug composition of the sodium ozagrel compound and a preparation method thereof. The dosage forms of the drug composition include freeze-dried powder injection, small-volume injection or large-volume injection. The sodium ozagrel compound has high purity and the preparations of the sodium ozagrel compound have good stability and are suitable for clinical application.
Owner:HAINAN LEVTEC PHARMA
Method for detecting content of bromo-succinimide and succinimide in sodium ozagrel raw material medicine
The invention discloses a method for detecting content of bromo-succinimide and succinimide in sodium ozagrel raw material medicine. The method comprises: taking a to-be-detected sodium ozagrel sample, and after adding aqueous solution of hydrochloric acid to dissolve, obtaining test solution; detecting succinimide in the test solution by utilizing a high-performance liquid chromatography technology, and obtaining total content of the bromo-succinimide and the succinimide in the sodium ozagrel raw material medicine. According to the method disclosed by the invention, by using the hydrochloricacid as a solvent, firstly the bromo-succinimide in the to-be-detected sodium ozagrel sample is totally converted into the succinimide, and then detection is carried out by adopting the liquid chromatography technology with specific parameter conditions, so that the total content of the bromo-succinimide and the succinimide in the sodium ozagrel raw material medicine can be effectively and accurately detected, the theoretical basis is provided for identification of impurities in the sodium ozagrel raw material medicine, and quality of the sodium ozagrel raw material medicine and downstream medicine are ensured.
Owner:浙江科瑞医药科技有限公司
Ozagrel compound, preparation method and pharmaceutical composition of ozagrel compound
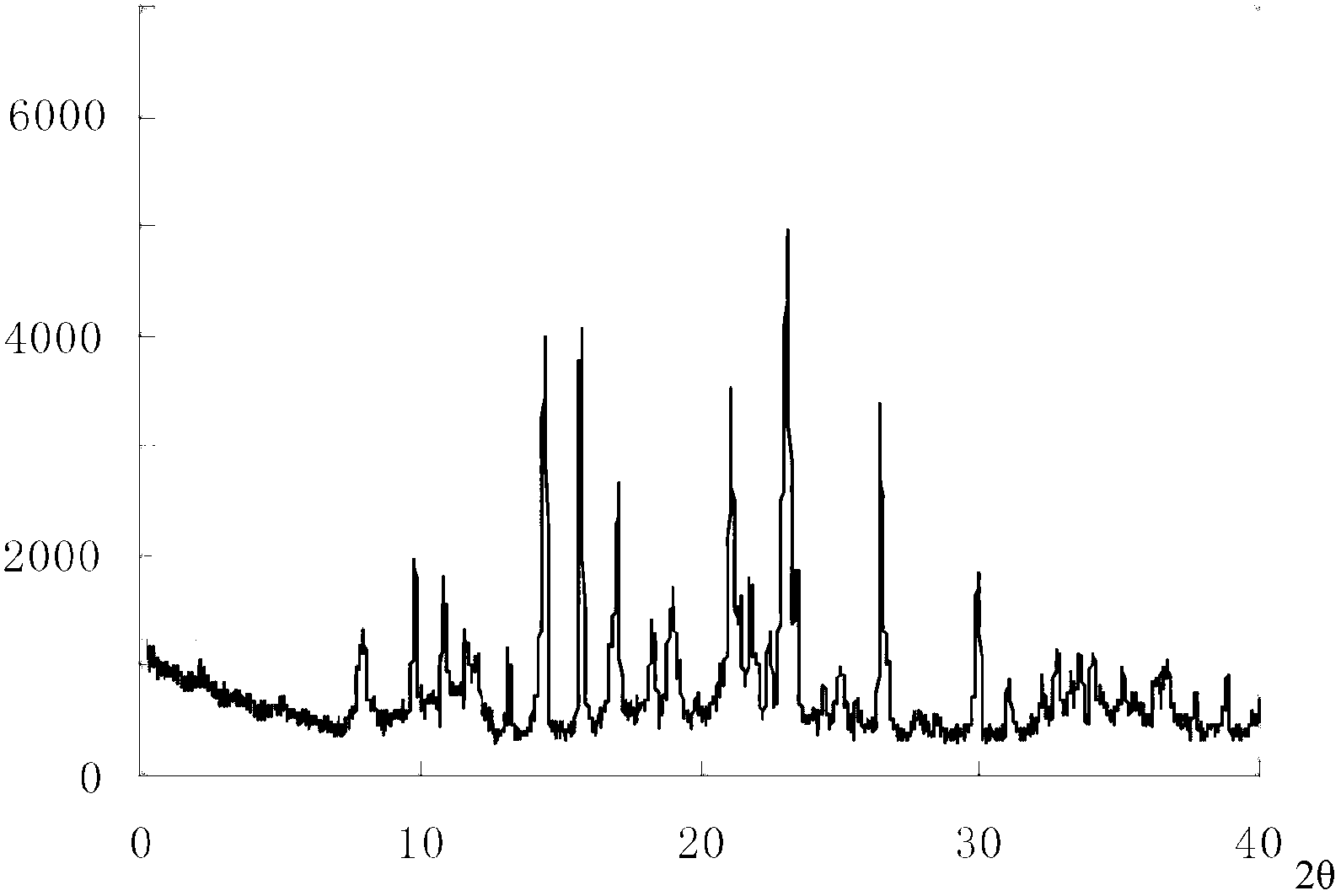
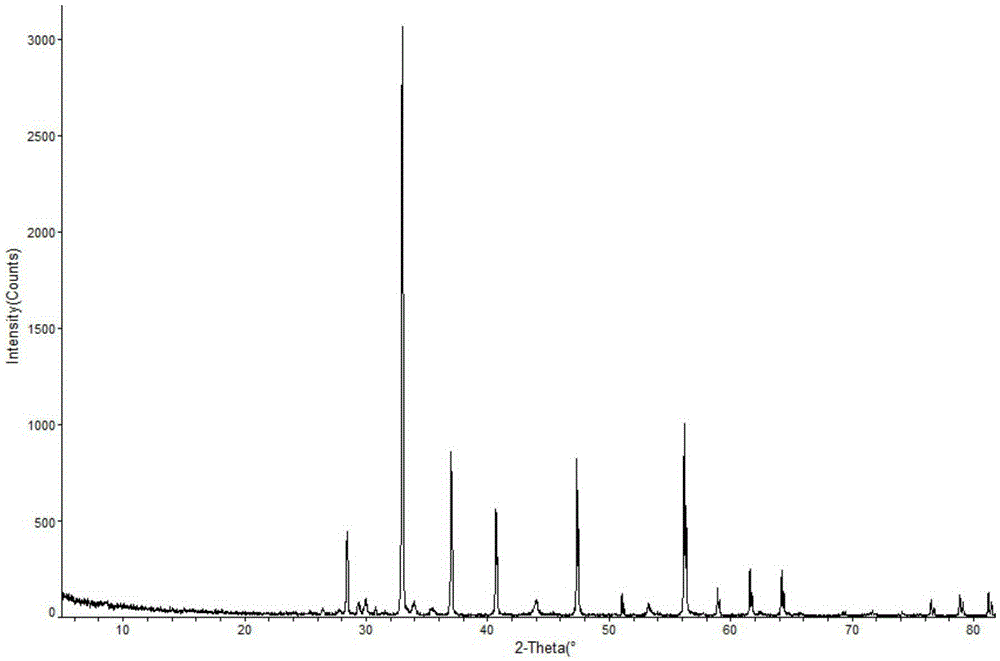
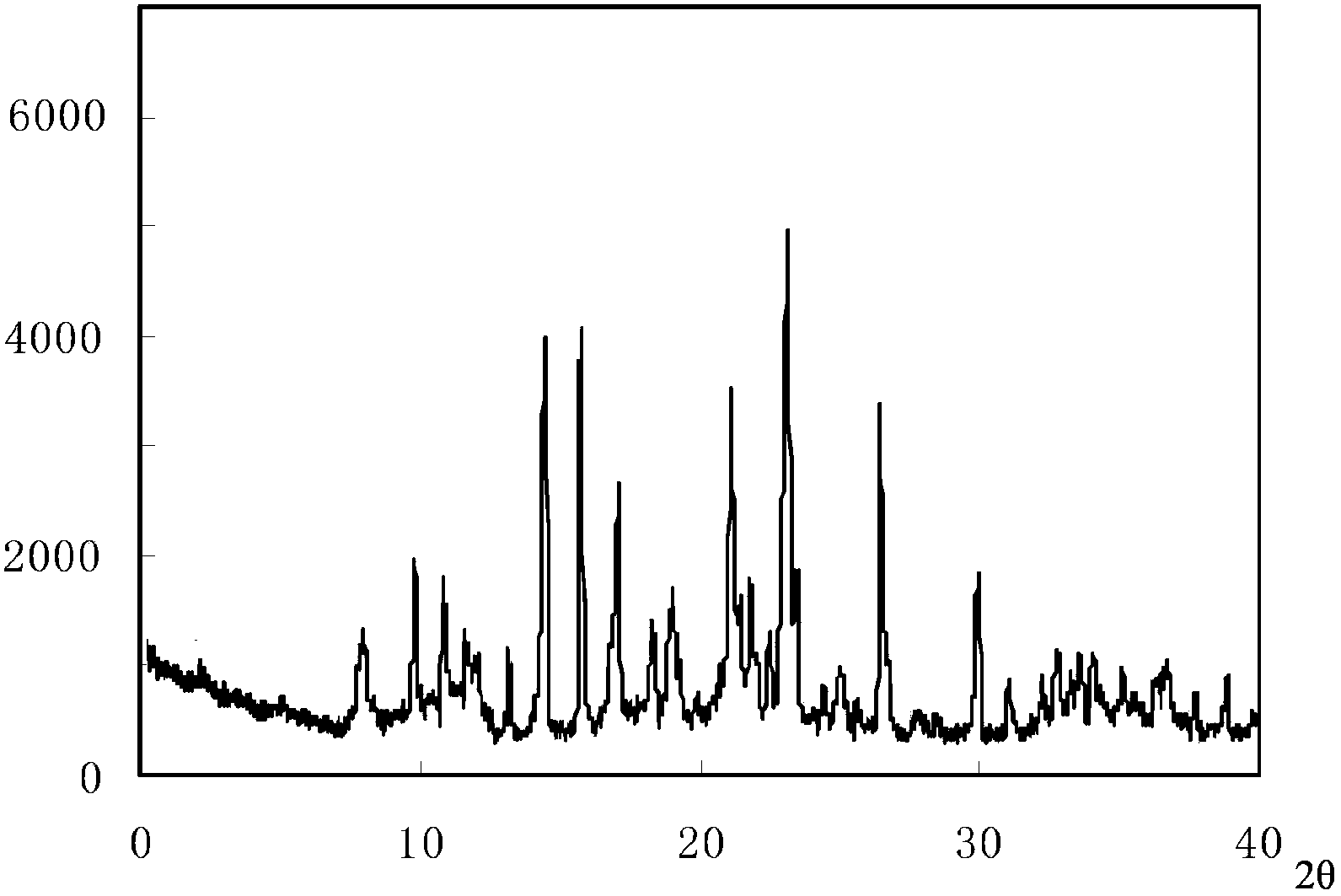
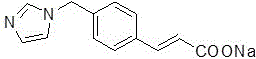
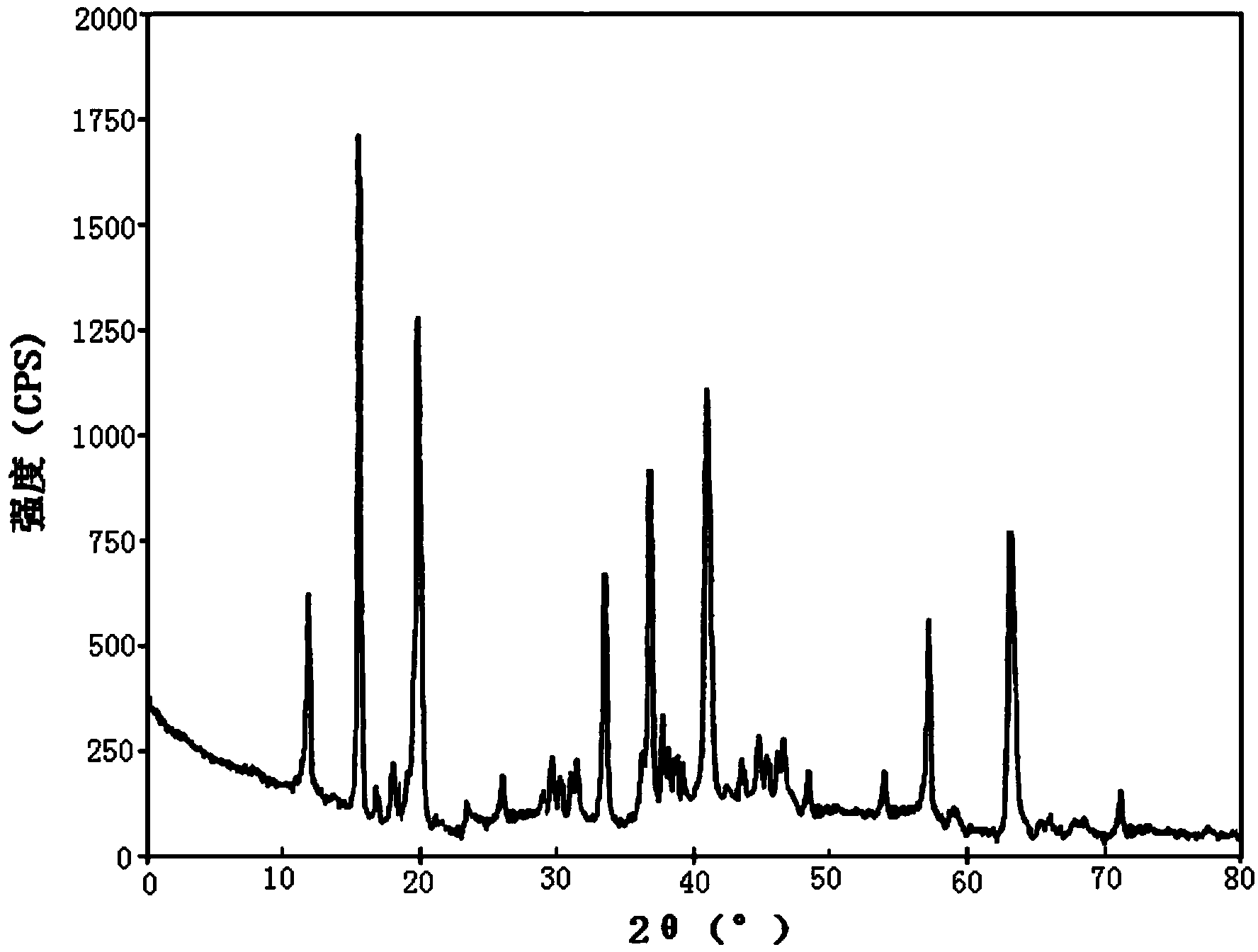
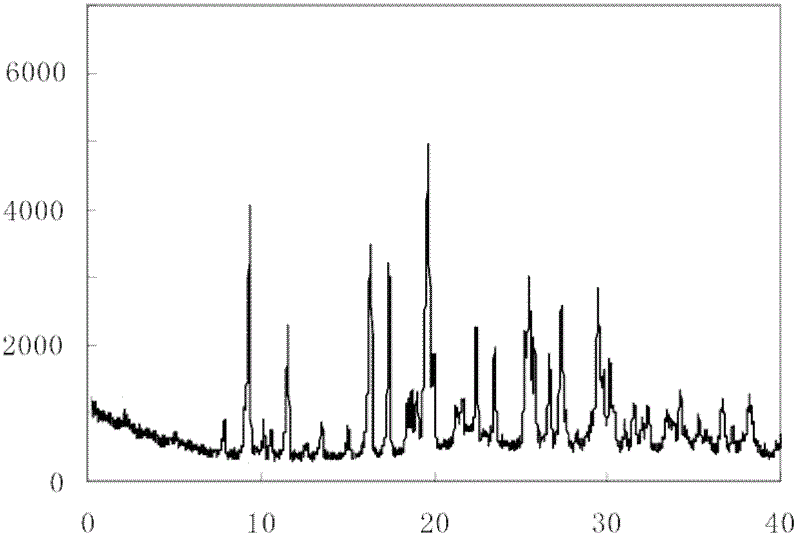
ActiveCN103450086ALittle changeReduce contentOrganic active ingredientsPowder deliveryCombinatorial chemistryOzagrel
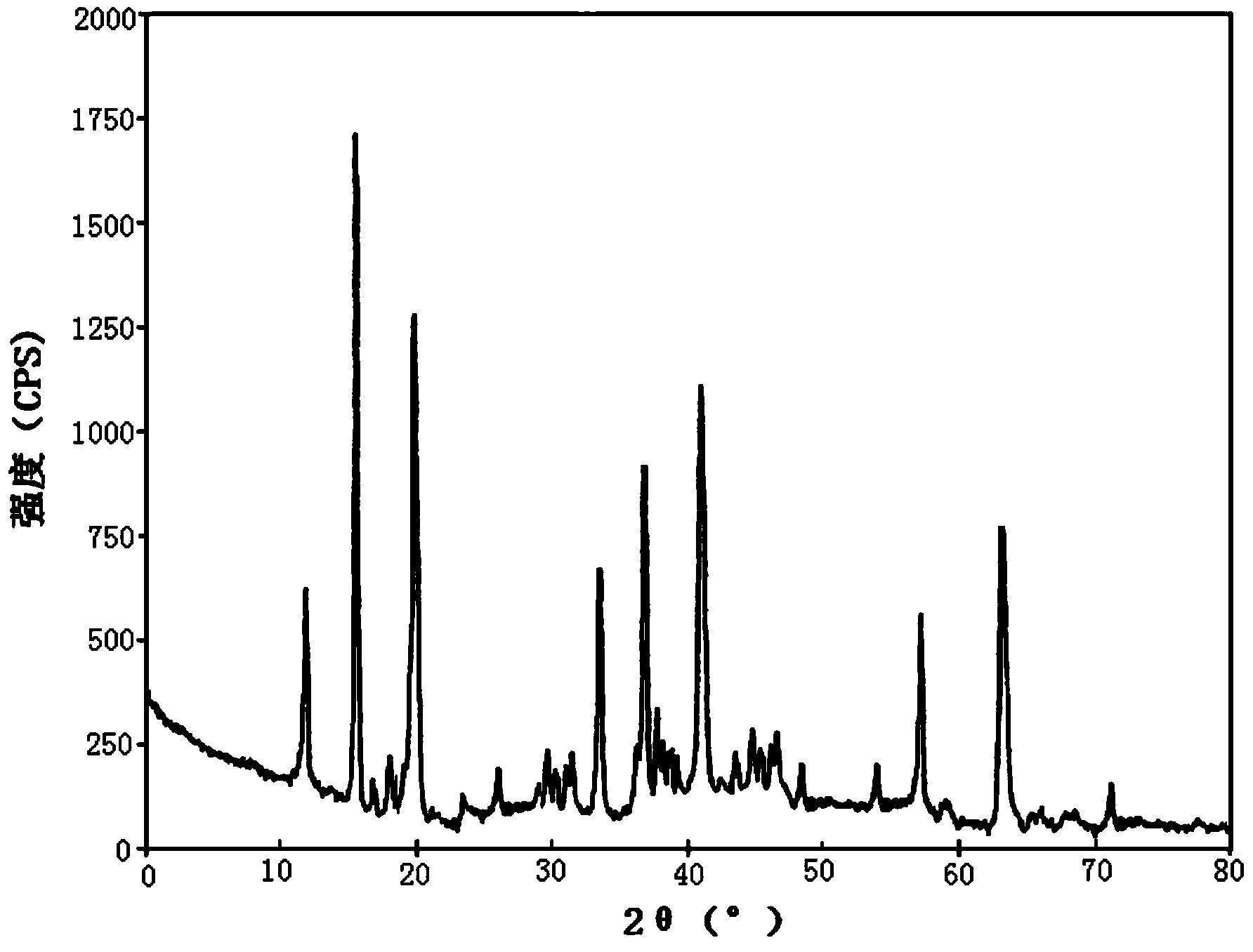
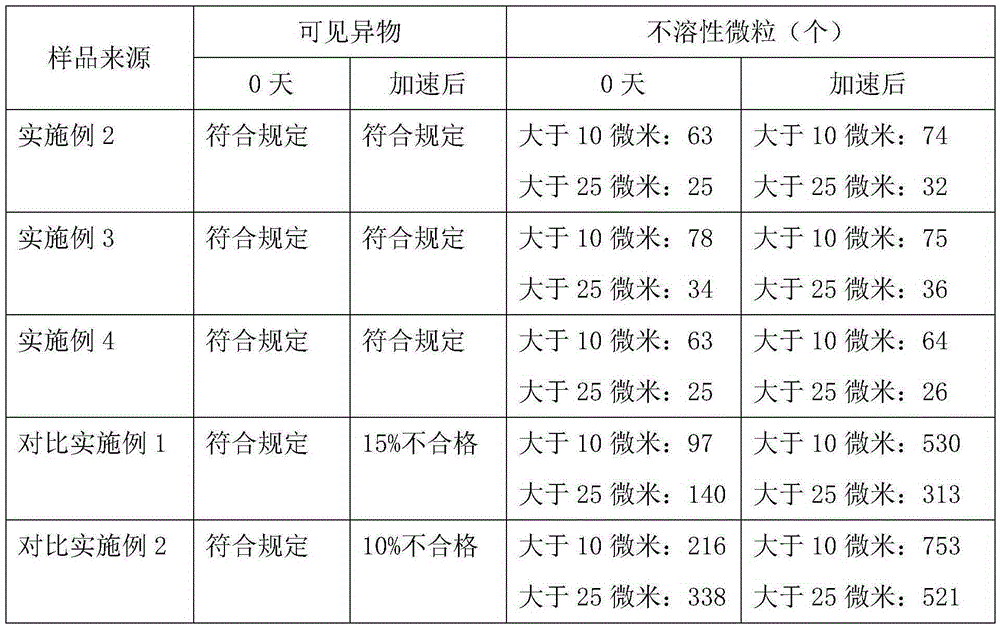
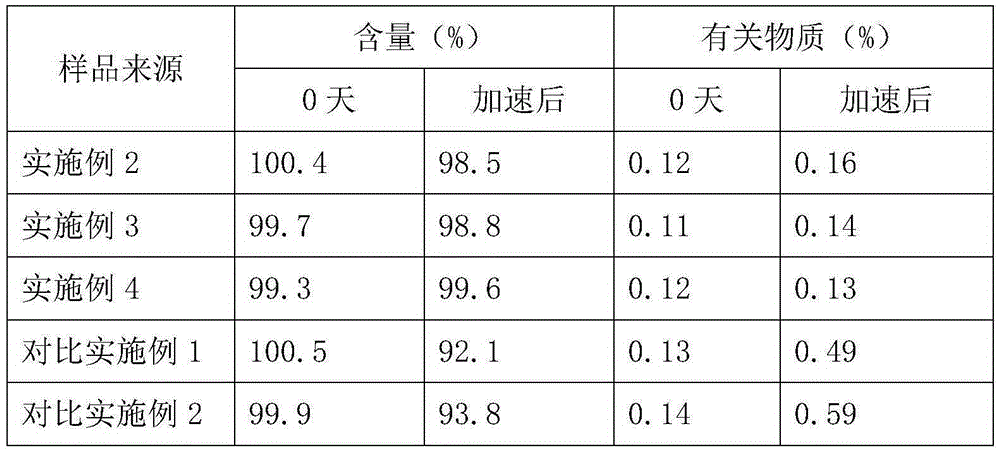
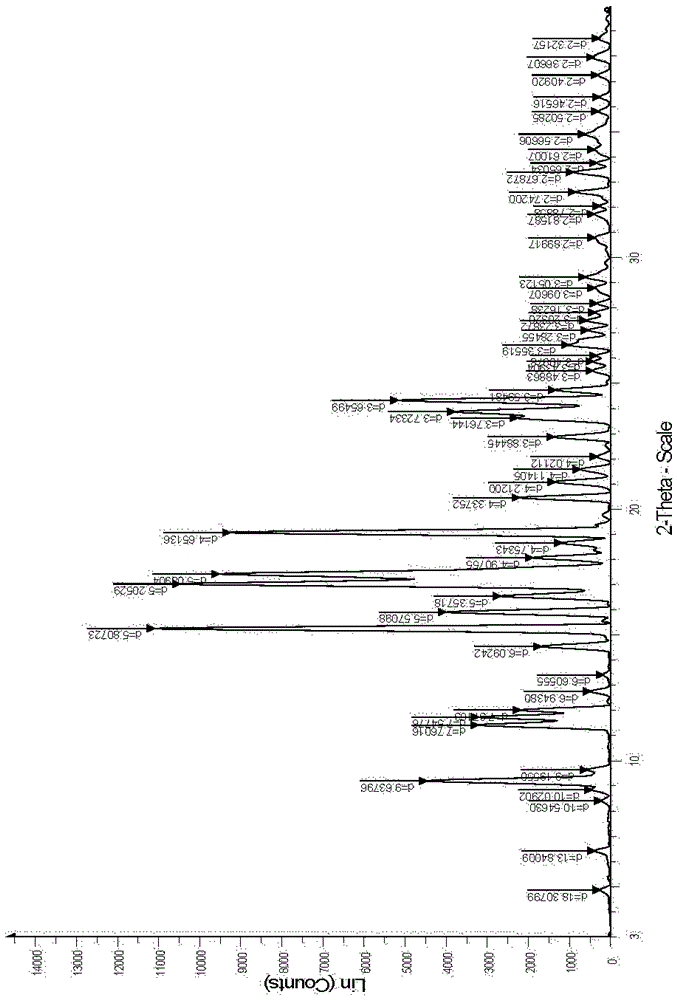
The invention belongs to the technical field of medicines, and particularly relates to an ozagrel compound, a preparation method and a pharmaceutical composition of the ozagrel compound. The X-ray powder diffraction spectrogram obtained by Cu-K alpha-ray measurement is shown in a figure 1; and the structural formula is as shown in a formula (I). The ozagrel compound is a novel crystal form which is different from that in the prior art. The novel crystal form ozagrel compound has humidity, temperature and illumination stability obviously superior to those of the ozagrel in the prior art; sodium ozagrel for injection, prepared from the ozagrel compound of such crystal form, is high in redissolving performance, and insoluble particles have smaller change after an injection solution is combined; after redissolving, the contents of specific impurities I and II in the solution are obviously less than those in the prior art.
Owner:北京科创鼎诚医药科技有限公司
Sodium ozagrel freeze-dried powder for injection and preparation method of sodium ozagrel freeze-dried powder
InactiveCN104352451AImprove qualityImprove stabilityOrganic active ingredientsPowder deliverySide effectMANNITOL/SORBITOL
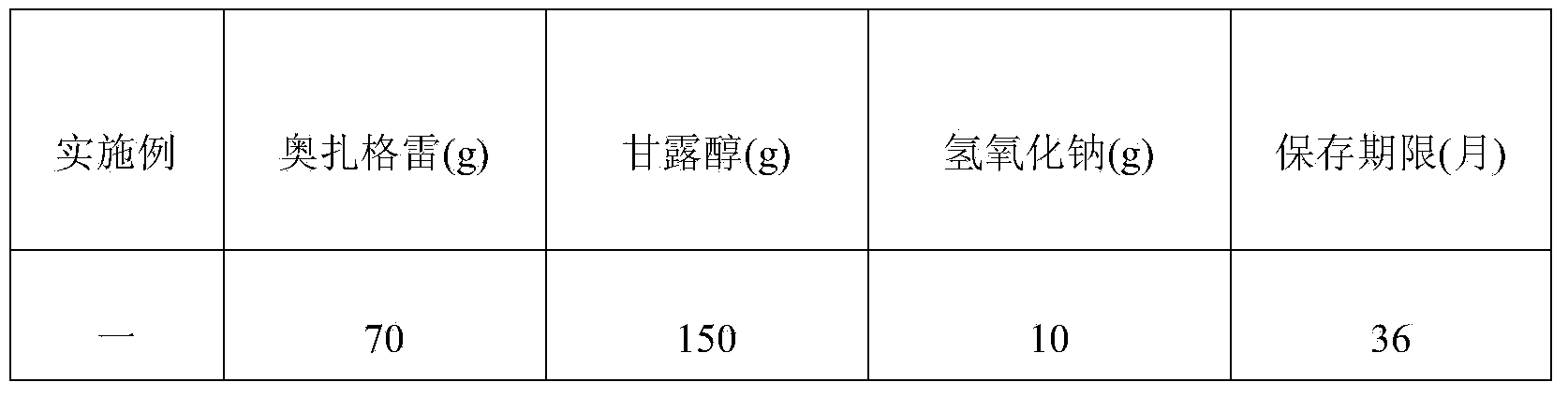
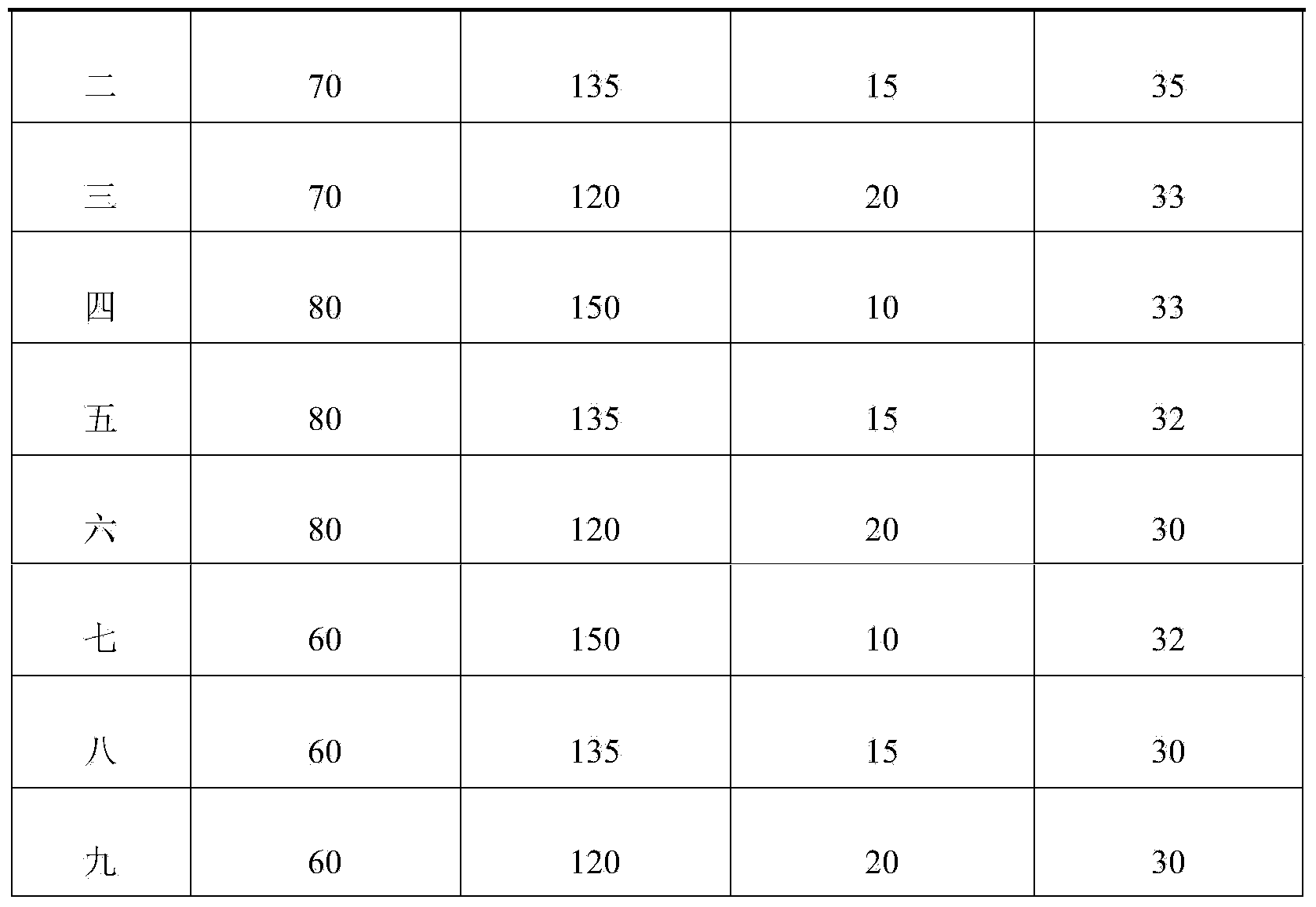
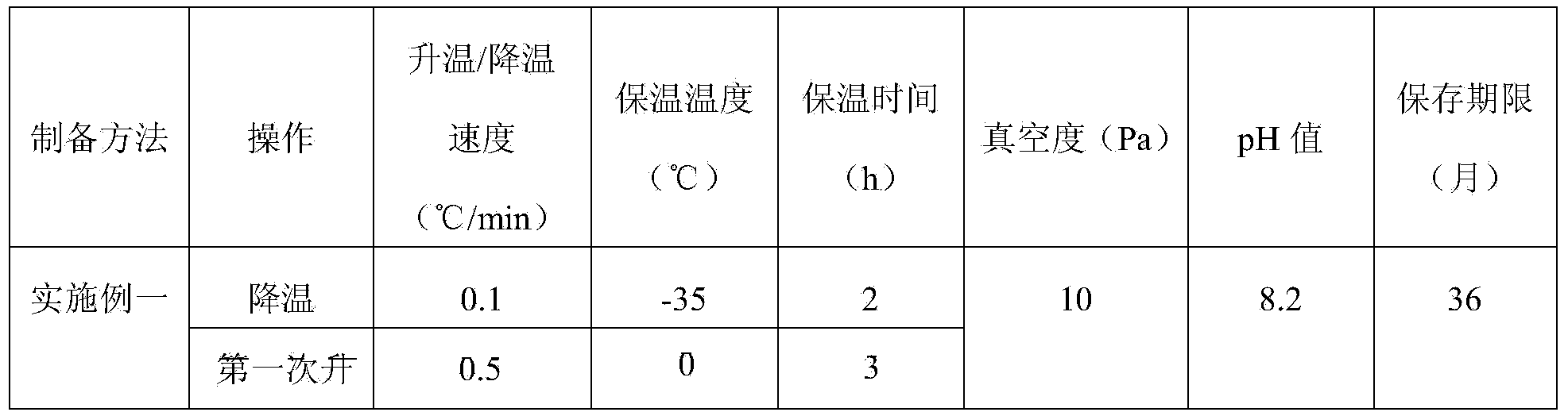
The invention provides sodium ozagrel freeze-dried powder for injection. The sodium ozagrel freeze-dried powder comprises formula components in parts by weight as follows: 60-80 parts of ozagrel, 120-150 parts of mannitol and 10-20 parts of sodium hydroxide. The sodium ozagrel freeze-dried powder has simple formula and fewer auxiliary materials and is applicable to popularization, side effects caused by excessive addition of auxiliary materials are avoided, and the safety of clinical use is improved. The invention further provides a preparation method of the sodium ozagrel freeze-dried powder for injection. Through accurate control on parameters such as the heating speed, the holding temperature, the holding time, the pressure and the like of freeze-drying process steps, the obtained sodium ozagrel freeze-dried powder for injection is more normative in preparation course, good in product quality and high in stability.
Owner:HAINAN GENERAL & KANGLI PHARMA
New preparation method of Ozagrel sodium powder injection
InactiveCN101596167ALittle side effectsConvenient for clinical operationOrganic active ingredientsPowder deliverySide effectCLARITY
The invention researches a new preparation method by aiming at the problems of side effect and inconvenient use of the clinical application of an Ozagrel sodium refrigerated dry powder injection and applies polyglucose to the Ozagrel sodium refrigerated dry powder injection for the first time, the Ozagrel sodium refrigerated dry powder injection is decolorized by active carbon and filtered by a filter film with the aperture of 0.22 micrometer so as to solve the poor appearance problem of the Ozagrel sodium powder injection, achieve the purposes of convenient use, cost reduction, curative effect guarantee and side effect reduction and solve the problem of unqualified clarity generated by applying the polyglucose to the refrigerated dry injection.
Owner:HARBIN PHARMA GROUP BIOLOGICAL ENG
Injection ozagrel sodium freeze-dried powder for treating cerebral infarction
InactiveCN105267162AImprove stabilityReduce typesOrganic active ingredientsPowder deliveryFreeze-dryingMicroparticle
The invention discloses injection ozagrel sodium freeze-dried powder for treating cerebral infarction, and belongs to the technical field of medicines. The ozagrel sodium is a crystal, and the novel crystal form of ozagrel sodium, provided by the invention, is different from a crystal structure of the prior art, and experiments prove that the novel crystal form compound is high in purity, good in fluidity and stability and low in impurity content, cannot absorb moisture easily, is safe and reliable in clinical application; and a powder injection prepared by using the novel crystal form compound is good in stability after being compatible with a solvent and extremely low in insoluble micro-particle content, and is very suitable for clinical application.
Owner:南京多宝生物科技有限公司
Method for preparing sodium ozagrel freeze-dried powder injection
ActiveCN105287408AControl contentImprove quality stabilityOrganic active ingredientsPowder deliveryFreeze-dryingOzagrel
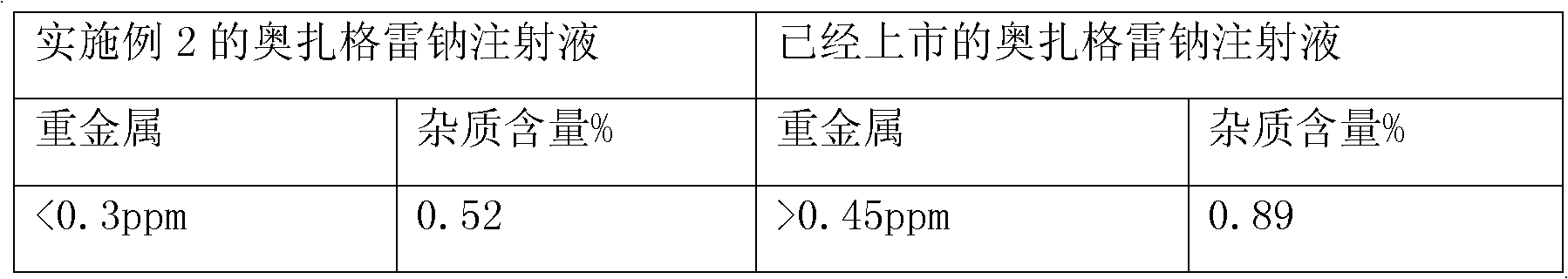
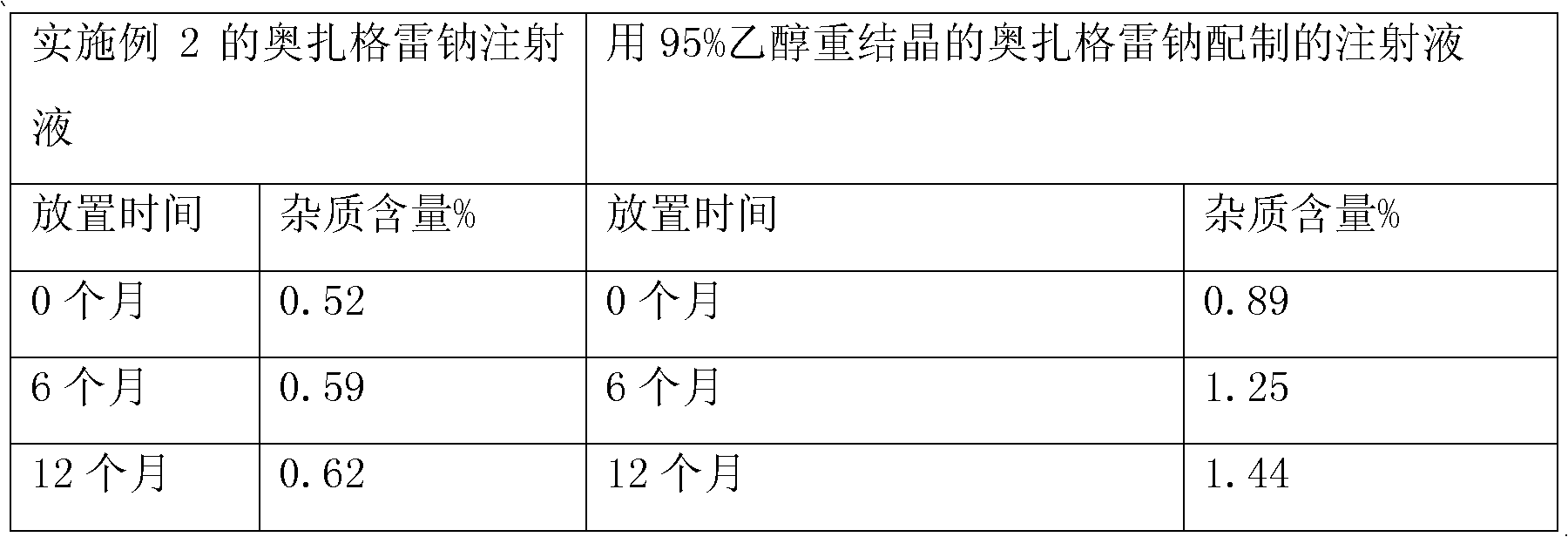
The invention provides a method for preparing a sodium ozagrel freeze-dried powder injection. The method comprises the following steps: a) mixing ozagrel, a sodion-containing alkaline matter and first injection water, and conducting pH value adjustment and adsorption treatment in sequence to obtain a mixed solution A; b) mixing the mixed solution A with second injection water, and conducting refrigeration and drying in sequence so as to obtain the sodium ozagrel freeze-dried powder injection; the volume ratio of the first injection water to the second injection water is 6:(3-5). Compared with the prior art, the provided preparation method can effectively control the content of impurities in the sodium ozagrel freeze-dried powder injection, and can improve the quality stability of the product. Experiment results show that the content of the impurities in the sodium ozagrel freeze-dried powder injection obtained by the provided preparation method is 0.3% or below.
Owner:HUNAN KELUN PHARMA
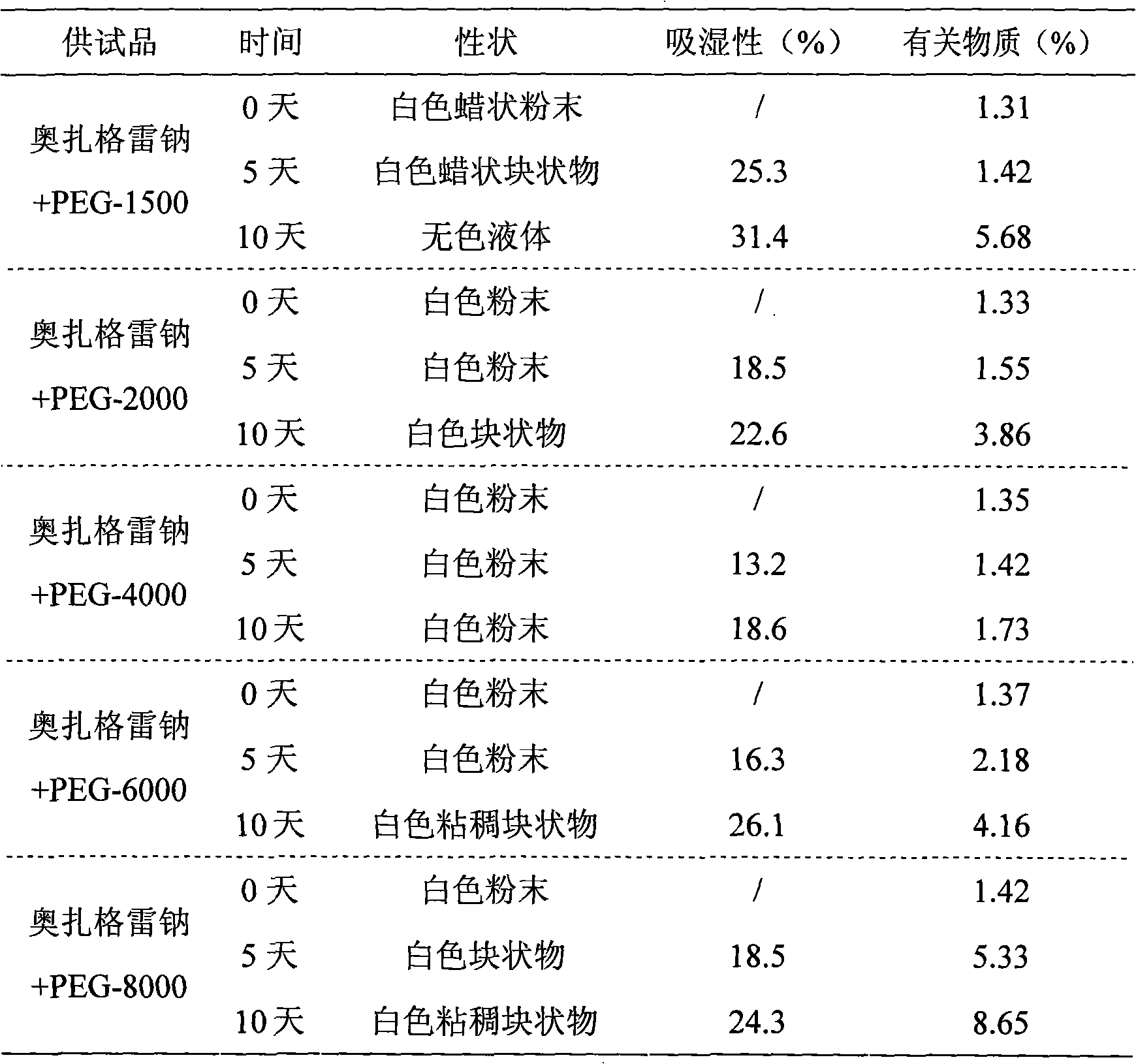
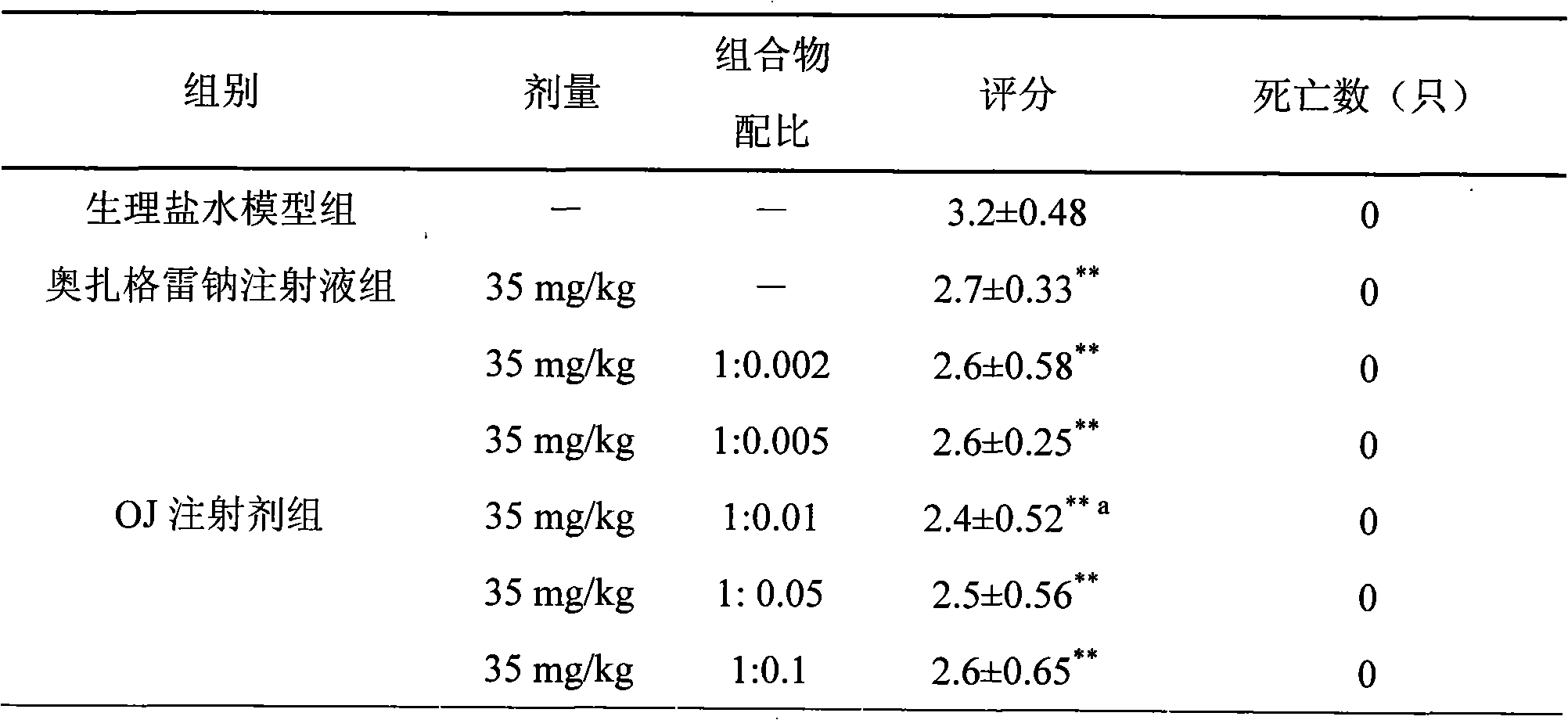
Composition of sodium ozagrel and polyethylene glycol and preparation method thereof
InactiveCN101780072AImprove effectivenessImprove in vivo stabilityOrganic active ingredientsPowder deliverySodium bicarbonateMANNITOL/SORBITOL
The invention discloses a composition of sodium ozagrel and polyethylene glycol and a preparation method thereof, belonging to the medical technical field. The weight ratio of sodium ozagrel to polyethylene glycol in the pharmaceutical composition is 1:0.002-0.1. Injections can be prepared by using the pharmaceutical composition and auxiliary material which is acceptable in pharmacy and the auxiliary material is selected from one or more of lactose, mannitol, sorbitol, dextran, citric acid-sodium citrate and sodium bicarbonate-sodium carbonate. The pharmaceutical composition is used to cure dyskinesia associated with acute thrombotic infarction and cerebral infarction.
Owner:BEIJING SIHUAN PHARMA +1
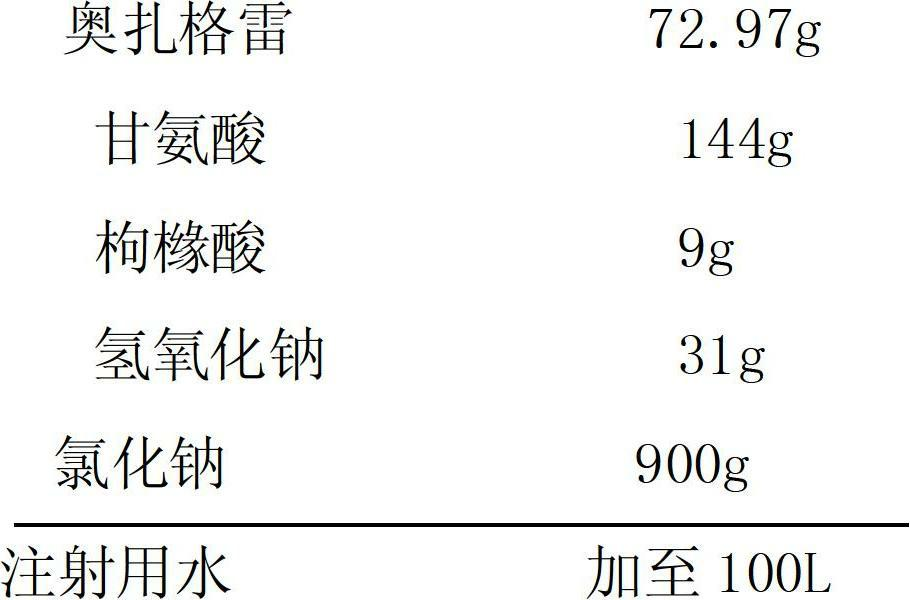
Sodium ozagrel sodium chloride injection and preparation method thereof
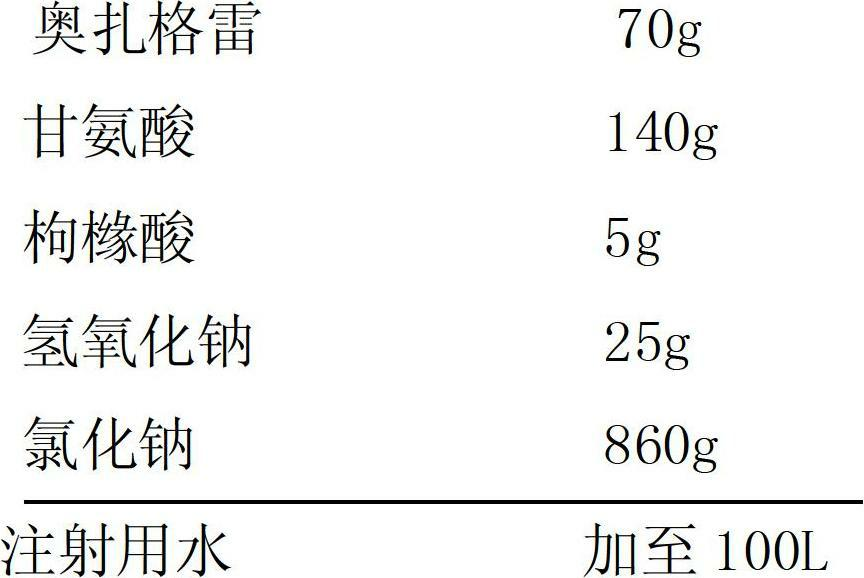
InactiveCN102670491AImprove stabilityNo decompositionOrganic active ingredientsInorganic non-active ingredientsGlycineSodium Chloride Injection
The invention provides a sodium ozagrel sodium chloride injection and a preparation method thereof and belongs to the technical field of medicines. The invention aims to overcome the defects that a traditional sodium ozagrel raw material is high in cost and cannot meet the market demand, and moreover, the preparation technology is complicated due to the fact that sodium ozagrel and the sodium ozagrel sodium chloride injection are separately prepared. The injection comprises the following components in parts by weight: 70-75 parts of ozagrel, 140-150 parts of glycine, 5-15 parts of citric acid, 25-35 parts of sodium hydroxide and 850-900 parts of sodium chloride. The invention also provides the preparation method for the sodium ozagrel sodium chloride injection. The sodium ozagrel sodium chloride injection provided by the invention is high in stability and is obtained by directly synthetizing the sodium ozagrel during the preparation process. The raw material is low in cost, the technical process is simple, and the cost is low.
Owner:CHANGCHUN HAOBANG PHARMA
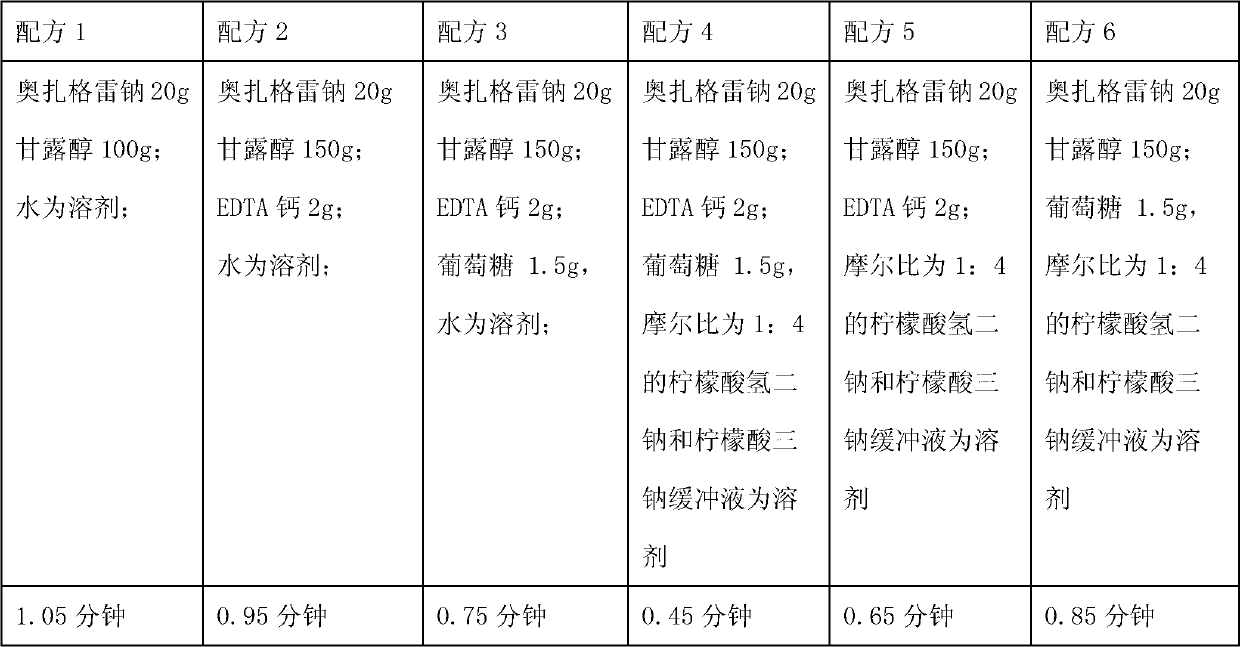
Medicinal composition containing sodium ozagrel compound
ActiveCN102988306AImprove stabilityImprove tolerancePowder deliveryOrganic active ingredientsEthylene diamineFreeze-drying
The invention relates to a medicinal composition containing sodium ozagrel, and the medicinal composition is a freeze-dried injection. Each 1,000 injections are prepared from the following components: 20g of sodium ozagrel, 100 to 200g of mannitol, 1 to 3g of ethylene diamine tetraacetic acid (EDTA) calcium, 1 to 2g of glucose, and 2,000ml of buffer solution of disodium hydrogen citrate and trisodium citrate at a mole ratio of 1: 4, wherein the pH value of the injection is 7.0.
Owner:姚云
Method for preparing ozagrel sodium crystal
ActiveCN101397272BHigh purityHigh yieldOrganic chemistryCardiovascular disorderMicrofiltration membraneTherapeutic effect
The invention relates to a simple technical process used for preparing high-pure ozagrel sodium crystal. The process basically includes the following steps: the raw ozagrel sodium is added into ethanol and then heated for dissolving; active carbon is added for decoloring and filtering is carried out under the heated condition; the filtrate is filtered in a refined way by a microfiltration membrane, collected, heated first and then cooled slowerly; and the temperature is controlled to be 25 DEG C to 60 DEG C for temperature-preservation crystallization and then the temperature is reduced slowly and cooled for crystallization; finally the crystal is collected. The crystal prepared by the method is high in purity and yielding and low in impurity content; therefore the therapy effect of the product is effectively enhanced and the cost is reduced simultaneously.
Owner:HAINAN BIKAI PHARM CO LTD
Method for detecting impurity elements in sodium ozagrel
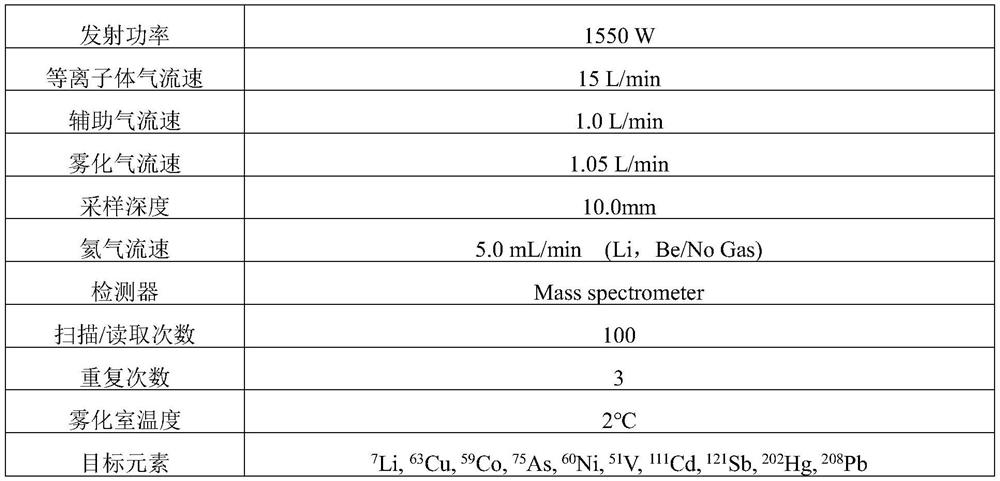
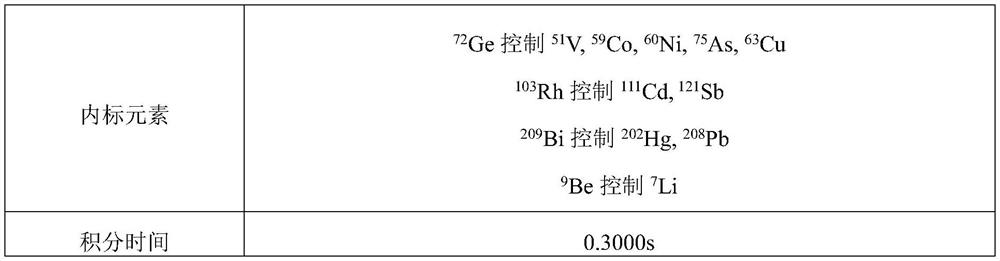
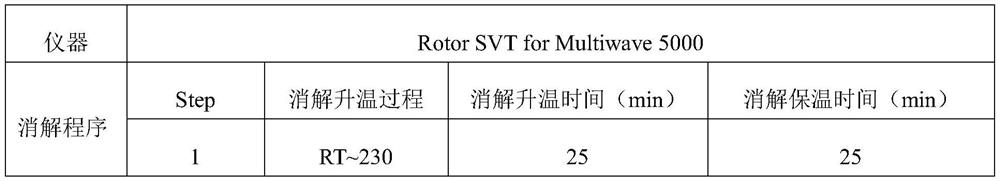
PendingCN114323874ADetection contentPreparing sample for investigationMaterial analysis by electric/magnetic meansInternal standardPhysical chemistry
The invention discloses a method for detecting impurity elements in sodium ozagrel. The detection method comprises the following steps: (1) testing the response ratio of impurity elements to internal standard elements in a reference substance solution by adopting inductively coupled plasma mass spectrometry, and obtaining a linear equation; (2) testing the response ratio of the impurity element to be tested to the internal standard element in the test solution, and substituting the response ratio into the linear equation; the target impurity elements comprise Li, Sb, Cu, V, Co, Ni, As, Cd, Hg and Pb; the concentration of the sample to be detected in the test solution is 0.1-0.4 mg / mL; the preparation method of the test solution comprises the following steps: digesting a mixed solution of a to-be-detected sample and nitric acid, and diluting, wherein the mass volume ratio of the to-be-detected sample to the nitric acid is 5-8 mg / mL. According to the detection method, the content of various impurity elements in sodium ozagrel can be rapidly, effectively and simultaneously detected, the stability is good, and the detection is efficient.
Owner:武汉九州钰民医药科技有限公司
Sodium ozagrel compound, preparation method and drug composition thereof
Owner:HAINAN LEVTEC PHARMA
Ozagrel sodium injection and preparation method thereof
ActiveCN101695475BQuality assuranceEnsure stabilityPowder deliveryOrganic active ingredientsMannitolPharmaceutical Aids
The invention relates to an ozagrel sodium injection and a preparation method thereof, in particular to an ozagrel sodium injection for treating acute thrombotic cerebral infarction and movement disorders concomitant with cerebral infarction, preferably injection solution and lyophilized powder injection. The ozagrel sodium injection mainly comprises ozagrel serving as an active component and sodium hydroxide and citric acid serving as auxiliary materials. Solvent in the injection solution is water for injection. Excipient in the lyophilized powder injection is mannitol and / or sorbitol which can be combined in any one or two optional medicinal proportions, or no excipient is added.
Owner:HAINAN LEVTEC PHARMA
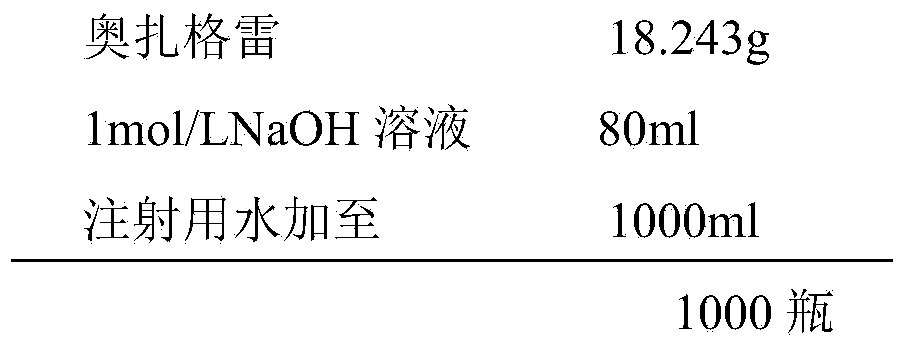
A kind of preparation method of ozagrel sodium freeze-dried powder injection
ActiveCN105287408BControl contentImprove quality stabilityPowder deliveryOrganic active ingredientsFreeze-dryingRefrigeration
The invention provides a method for preparing a sodium ozagrel freeze-dried powder injection. The method comprises the following steps: a) mixing ozagrel, a sodion-containing alkaline matter and first injection water, and conducting pH value adjustment and adsorption treatment in sequence to obtain a mixed solution A; b) mixing the mixed solution A with second injection water, and conducting refrigeration and drying in sequence so as to obtain the sodium ozagrel freeze-dried powder injection; the volume ratio of the first injection water to the second injection water is 6:(3-5). Compared with the prior art, the provided preparation method can effectively control the content of impurities in the sodium ozagrel freeze-dried powder injection, and can improve the quality stability of the product. Experiment results show that the content of the impurities in the sodium ozagrel freeze-dried powder injection obtained by the provided preparation method is 0.3% or below.
Owner:HUNAN KELUN PHARMA
Pharmaceutical composition with function of treating cerebrovascular diseases
The invention belongs to the technical field of medicine and particularly relates to a pharmaceutical composition with function of treating cerebrovascular diseases. The pharmaceutical composition contains ozagrel sodium and 1, 3-diaza-2, 4-cyclopentadiene, wherein the weight percentage of the ozagrel sodium is more than or equal to 95 percent and less than 100 percent, and the weight percentage of the 1, 3-diaza-2, 4-cyclopentadiene is more than zero and less than or equal to 5 percent. The pharmaceutical composition provided by the invention is determined by pharmacology experiment and toxicology experiment.
Owner:HAINAN BIKAI PHARM CO LTD
Double-ingredient sodium ozagrel freeze-dried powder injection solution for injection
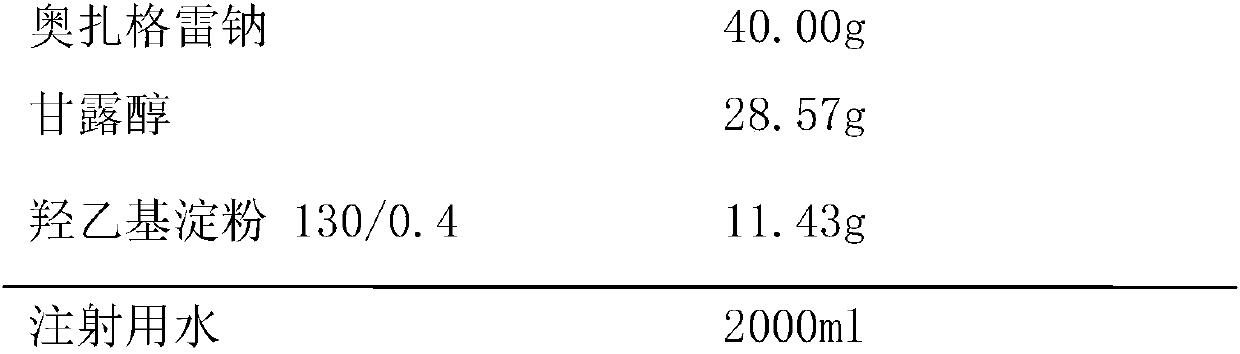
InactiveCN107714660ASimple preparation processImprove product qualityPowder deliveryOrganic active ingredientsHydroxyethyl starchMedicine
The invention provides a ozagrel sodium freeze-dried powder for injection with double auxiliary materials, relates to the field of pharmaceutical preparations and preparation methods, and mainly solves the problem of existing production of excipients in the production formula of ozagrel sodium freeze-dried powder for injection in the prior art The disadvantages of long freeze-drying time, high drying temperature and high energy consumption. The ozagrel sodium freeze-dried powder for injection uses double excipients, the excipients include mannitol and hydroxyethyl starch 130 / 0.4, the main drug is ozagrel sodium, and the weight ratio of the main drug to the excipients is 100:100‑100:140 , the weight ratio between mannitol and hydroxyethyl starch 130 / 0.4 is 100:30‑100:50. The ozagrel sodium freeze-dried powder for injection is prepared by using the above-mentioned main drug and auxiliary materials. The ozagrel sodium freeze-dried powder for injection provided by the invention is stable in quality, safe and effective, simple in production process, short in freeze-drying time during production, low in drying temperature and low in energy consumption.
Owner:刘兴付
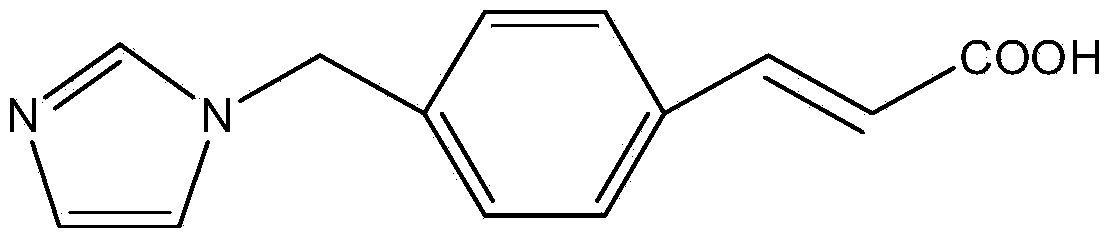
Ozagrel sodium crystal type compound
The invention belongs to the technical field of medicine, and particularly relates to an ozagrel sodium crystal type compound shown in a formula I. An X-ray powder diffraction pattern, obtained through Cu-K alpha ray measurement, of the compound is shown in figure 1. According to the ozagrel sodium crystal type compound, the bioavailability is improved.
Owner:ZHENGZHOU SIHUAN MEDICINE ARTICLE CO LTD
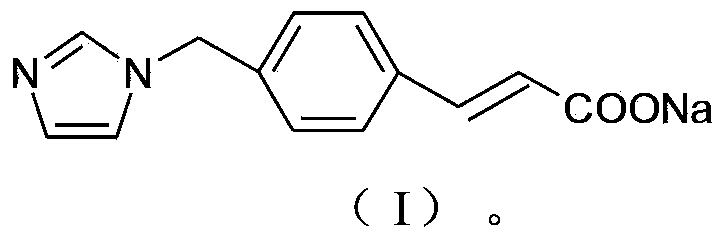
Ozagrel compound, preparation method and pharmaceutical composition of ozagrel compound
ActiveCN103450086BIncrease humidityImprove stabilityPowder deliveryOrganic active ingredientsCombinatorial chemistryMicroparticle
The invention belongs to the technical field of medicines, and particularly relates to an ozagrel compound, a preparation method and a pharmaceutical composition of the ozagrel compound. The X-ray powder diffraction spectrogram obtained by Cu-K alpha-ray measurement is shown in a figure 1; and the structural formula is as shown in a formula (I). The ozagrel compound is a novel crystal form which is different from that in the prior art. The novel crystal form ozagrel compound has humidity, temperature and illumination stability obviously superior to those of the ozagrel in the prior art; sodium ozagrel for injection, prepared from the ozagrel compound of such crystal form, is high in redissolving performance, and insoluble particles have smaller change after an injection solution is combined; after redissolving, the contents of specific impurities I and II in the solution are obviously less than those in the prior art.
Owner:北京科创鼎诚医药科技有限公司
Ozagrel sodium microballoon lyophilized preparation and preparation method thereof
InactiveCN101444491BImprove stabilityEvenly dispersedOrganic active ingredientsPowder deliverySolubilityPolyvinyl alcohol
The invention relates to an ozagrel sodium microballoon lyophilized preparation and a preparation method thereof. The ozagrel sodium microballoon lyophilized preparation of the invention mainly includes the following components by weight part: 20-80 parts of ozagrel sodium, 50-150 parts of chitosan, 40-100 parts of polyvinyl alcohol, and 50-300 parts of lyophilization protective agent. The ozagrelsodium microballoon lyophilized preparation of the invention has the advantages of good stability and solubility, small grain diameter of lipidosome after hydration, strong targeting property, low production cost and the like.
Owner:HAINAN MEIDA PHARMA
New ozagrel sodium compound and medicinal composition thereof
The invention relates to a new ozagrel sodium compound and a medicinal composition thereof. Ozagrel sodium is a crystal which is obtained by measuring by using u-K alpha rays and has characteristic peaks at 2 theta of 8.7 degrees, 10.3 degrees, 12.4 degrees, 16.2 degrees, 16.8 degrees, 19.2 degrees, 20.0 degrees, 20.7 degrees, 22.0 degrees, 22.9 degrees and 23.7 degrees during X-ray powder diffraction. The invention also relates to the medicinal composition for the ozagrel sodium compound. The medicinal composition comprises a freeze-dried powder injection or a water injection. The prepared freeze-dried powder injection has a good form, and is high in redissolution and stability, and the state of the prepared water injection is good and the prepared water injection has high stability.
Owner:周晓东
Thrombosis resisting composition
InactiveCN1199690CGood effectImprove solubilityAnthropod material medical ingredientsPeptide/protein ingredientsDiseaseSide effect
The thrombosis resisting composition consists of thromboxane A2(TXA2) synthetase inhibitor, thrombolytic medicine and / or thrombocyte resisting medicine; and can dissolving thrombus. The composition is obviously superior to single thromboxane A2(TXA2) synthetase inhibitor, and has high effect of preventing and treating thrombus diseases, small dosage and less side effect. Both extracorporeal and intracorporeal experiments shows that the composition has excellent direct thrombus dissolving effect and thrombocyte aggregation inhibiting effect. When the composition of sodium Ozagrel is used in treating acute cerebral thrombus, the clinical total effective rate is 100% and side effect originating rate is 2.1%. While only sodium Ozagrel is used, the corresponding data is 49.2% and 7.6% separately.
Owner:田兆军
Method for determining acetate in sodium ozagrel raw material medicine
PendingCN114371250AHigh densityIncreased durabilityComponent separationAcetic acidIon chromatography
The invention discloses a method for determining acetate in a sodium ozagrel raw material medicine. The determination method comprises the following step: detecting by adopting an ion chromatography method, wherein the chromatographic conditions are as follows: the chromatographic column is Thermo Dionex IonPac AS19, the column temperature is 20-30 DEG C, and the column temperature is 20-30 DEG C; the mobile phase is a KOH solution; the gradient elution is divided into three steps: the first step is that the KOH concentration is maintained to be 8-12 mmol / L within 0-(7-12) min; in the second step, the KOH concentration is maintained to be 30 mmol / L within the time from (7.1-12.1) min to (12-20) min; and in the third step, the concentration of KOH is maintained to be 8-12 mmol / L within the time range from (12.1-20.1) min to (14-30) min. According to the determination method disclosed by the invention, the content of acetate in sodium ozagrel can be accurately detected under given conditions.
Owner:武汉九州钰民医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com