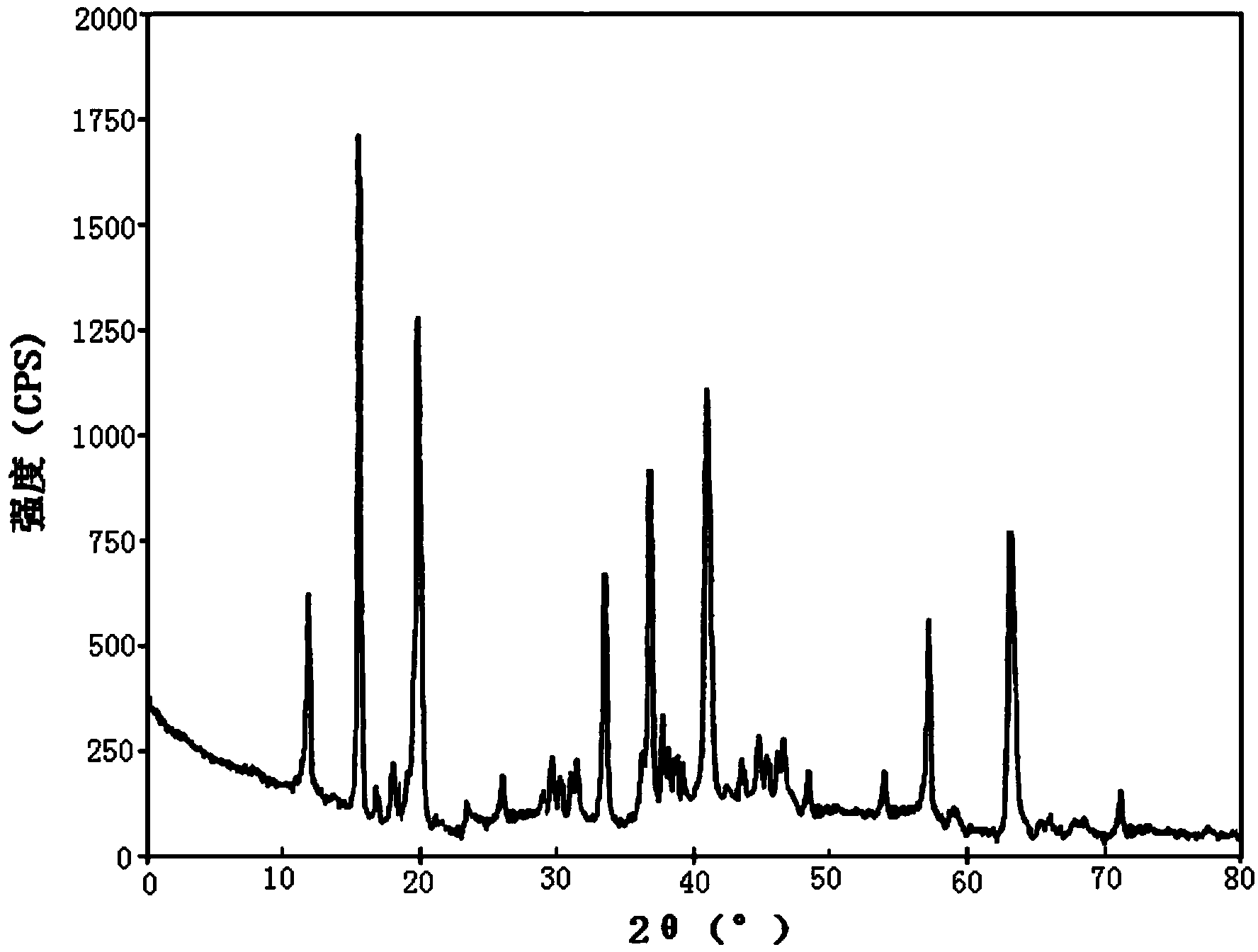
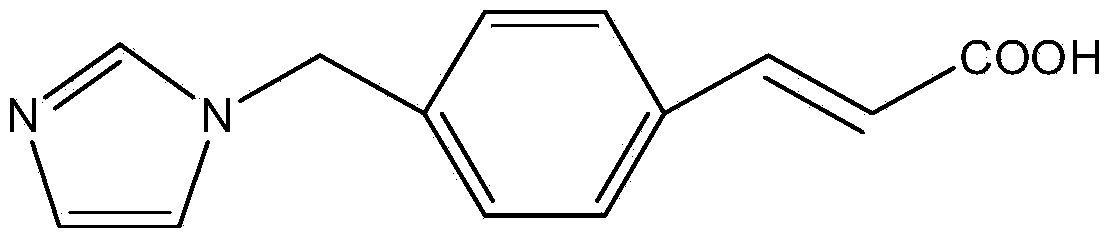
Ozagrel compound, preparation method and pharmaceutical composition of ozagrel compound
A compound and composition technology, applied in the field of medicine, can solve the problems of local circulation disorders, tissue hypoxia, allergic reactions, etc., and achieve the effects of good resolubility, little change in insoluble particles, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] [Example 1] Ozagrel compound
[0059] 1) Prepare mixed solution A by mixing dimethylformamide and methanol at a volume ratio of 1:4;
[0060] 2) Take the raw drug of ozagrel, and add the mixed solution A prepared in step 1) under an ultrasonic field with a power of 0.6KW, wherein the ratio of the volume of the mixed solution A to the mass of the raw drug of ozagrel is 6ml: 1g; after all dissolved, turn off the ultrasonic field, add 0.1% g / ml activated carbon to the obtained solution for decolorization, and filter to obtain a clear solution;
[0061] 3) Prepare mixed solution B with tetrahydrofuran and chloroform at a volume ratio of 1:3.5;
[0062] 4) At room temperature, add mixed solution B to the clear solution obtained in step 2) at a stirring speed of 850r / min, where the amount of mixed solution B added is 8 times the volume of mixed solution A, and cool down after adding After standing at 3°C for 2.5 hours, crystals were precipitated and dried to obtain the oz...
preparation Embodiment 1
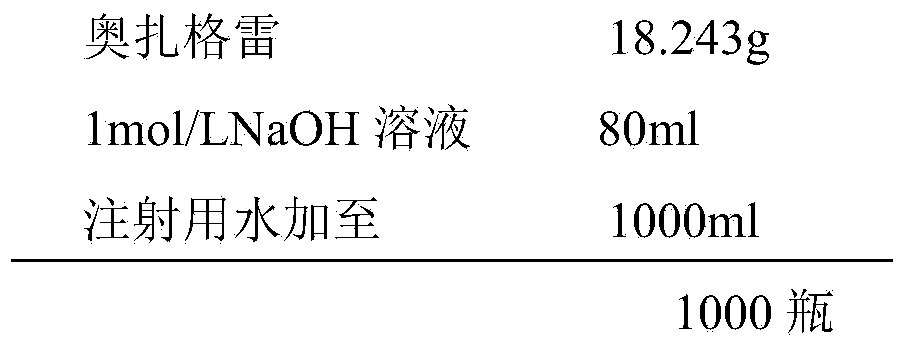
[0070] [Preparation Example 1] Sodium Ozagrel for Injection
[0071] Specification: 20mg (calculated as ozagrel sodium)
[0072] prescription:
[0073]
[0074] Preparation Process:
[0075] (1) Weigh the prescribed amount of ozagrel prepared in Example 1, add water for injection with a volume of 40% of the prescribed amount, add dropwise the prescribed amount of 1moL / L sodium hydroxide solution to dissolve the main ingredient, and add water for injection to the prescription Measure 80% of the volume, adjust the pH to about 9.0 with 1mol / L sodium hydroxide, add 0.1% (g / ml) activated carbon for room temperature adsorption, stir for about 20 minutes, decarbonize and filter, add water for injection to the total amount , sterile filtration;
[0076] (2) Intermediate determination (pH value and content) of the filtrate;
[0077] (3) Subpackage after passing the test, adopt quick-freezing method, and freeze-dry; the freeze-drying adopts the following process: the freeze drye...
preparation Embodiment 2
[0079] [Preparation Example 2] Sodium Ozagrel for Injection
[0080] Specification: 40mg (calculated as ozagrel sodium)
[0081] prescription:
[0082]
[0083] Preparation process: same as formulation example 1, except that the ozagrel used is the ozagrel prepared in example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com