Composition of sodium ozagrel and polyethylene glycol and preparation method thereof
A technology of ozagrel sodium and polyethylene glycol, which is applied in the field of medicine, can solve problems such as poor stability, limited dosage, and easy precipitation, and achieve improved stability in vivo, improved effectiveness, and good brain targeting Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
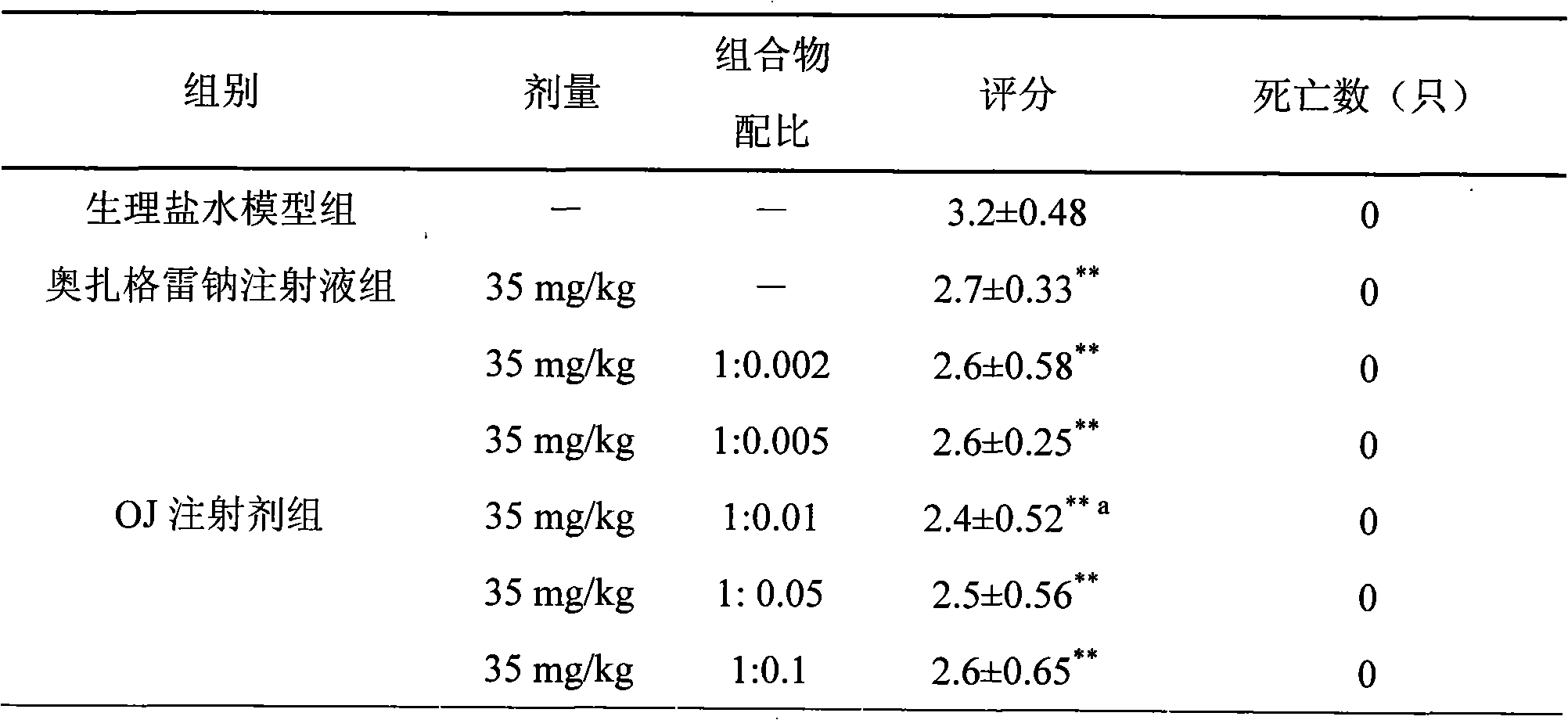
Examples
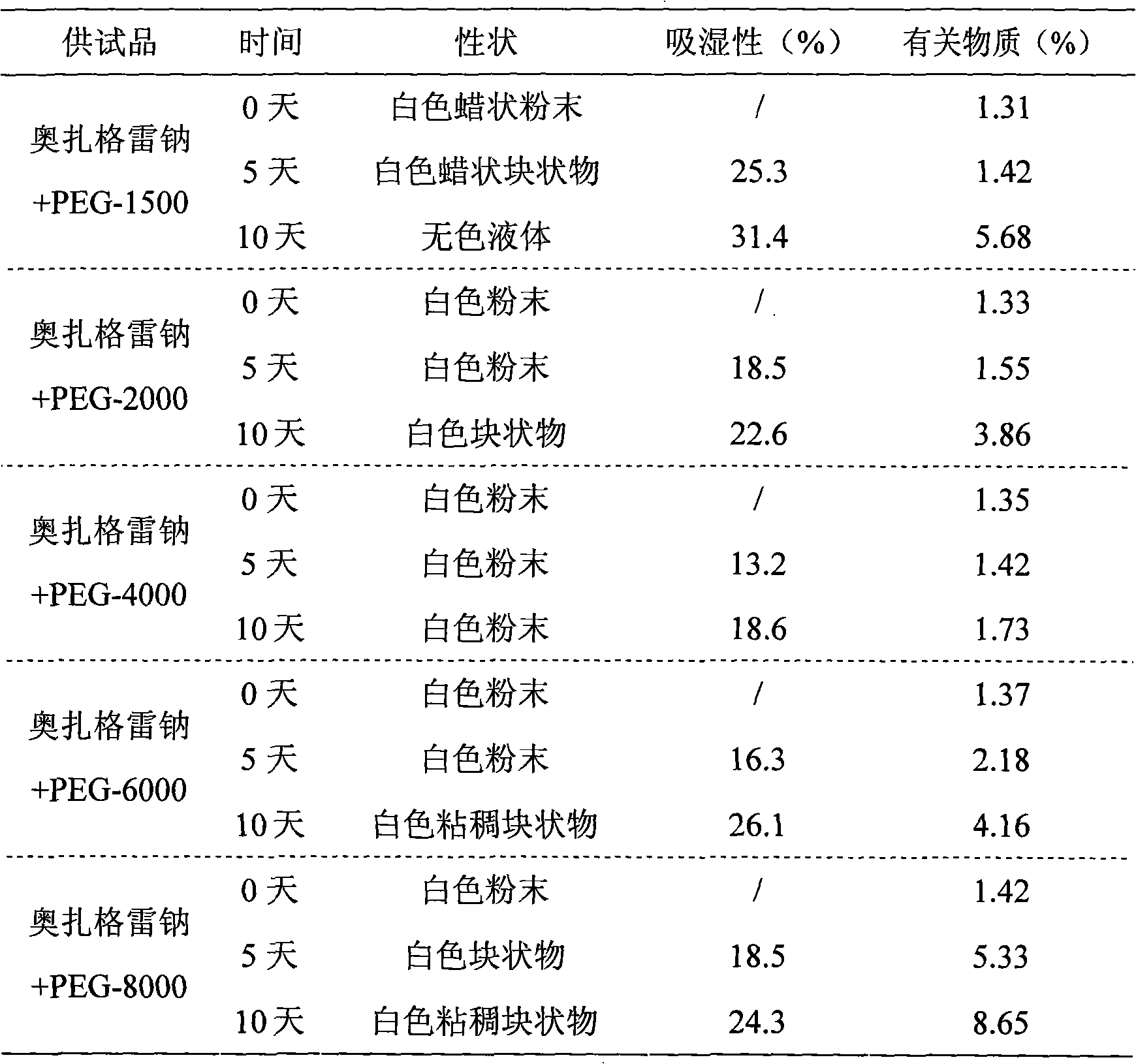
Embodiment O
[0060] The preparation of embodiment OJ freeze-dried powder preparation
[0061] 1. Prescription
[0062] Prescription 1:
[0064] PEG-40000.008g
[0065] Mannitol 4g
[0066] Add water for injection to 1000mL
[0067]
[0068] A total of 1000 bottles were made
[0069] Prescription 2:
[0070] Sodium Ozagrel 20g
[0071] PEG-4000 0.02g
[0072] Mannitol 4g
[0073] Add water for injection to 1000mL
[0074]
[0075] A total of 1000 bottles were made
[0076] Prescription 3:
[0077] Sodium Ozagrel 40g
[0078] PEG-40000.08g
[0079] Mannitol 8g
[0080] Add water for injection to 1000mL
[0081]
[0082] A total of 1000 bottles were made
[0083] Prescription 4:
[0084] Sodium Ozagrel 40g
[0085] PEG-40000.4g
[0086] Mannitol 8g
[0087] Add water for injection to 1000mL
[0088] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com