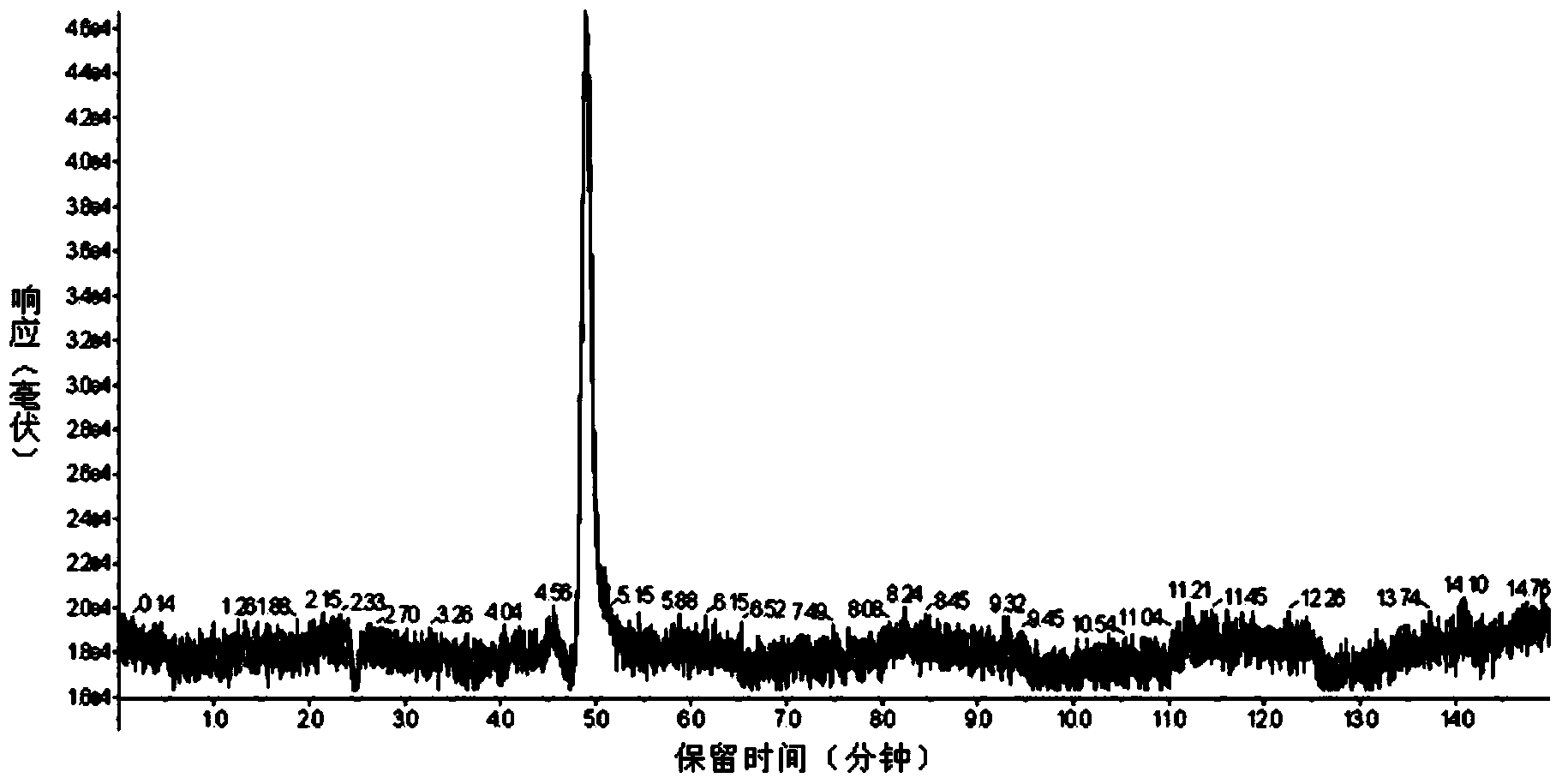
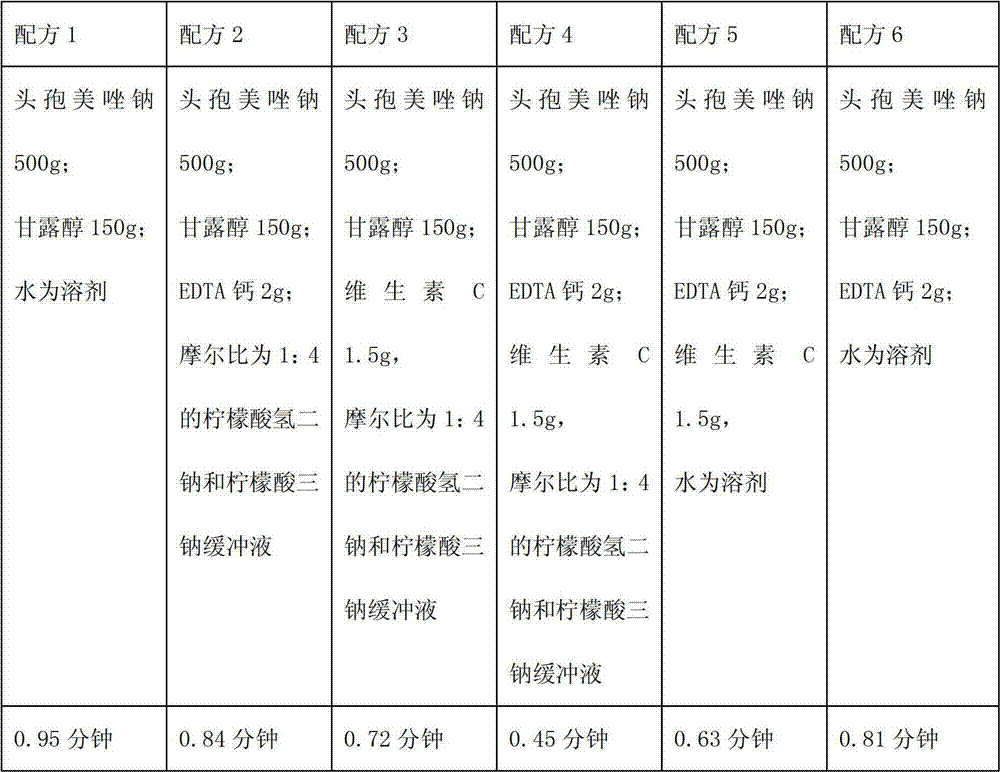
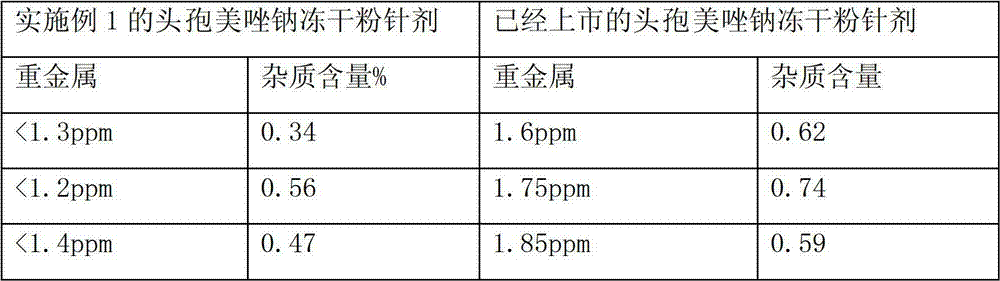
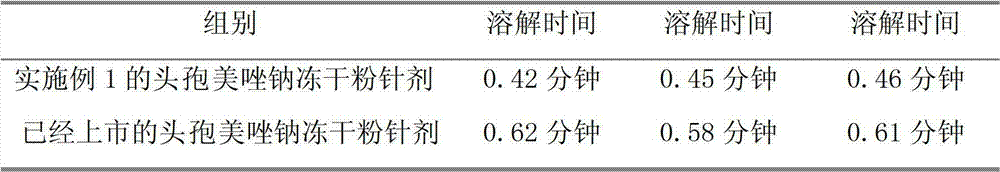
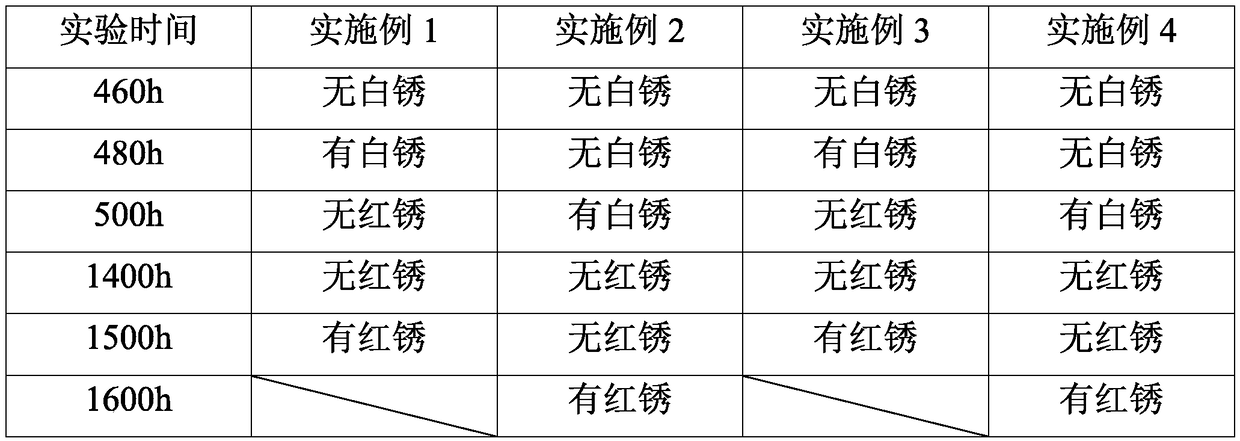
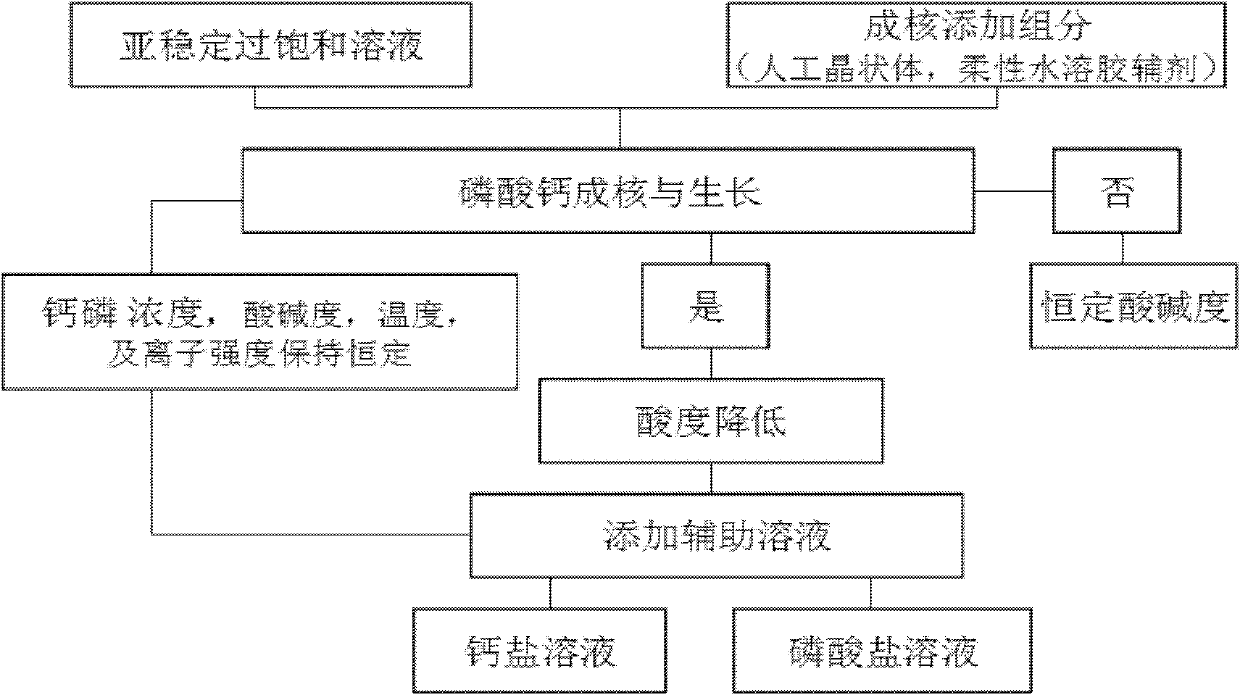
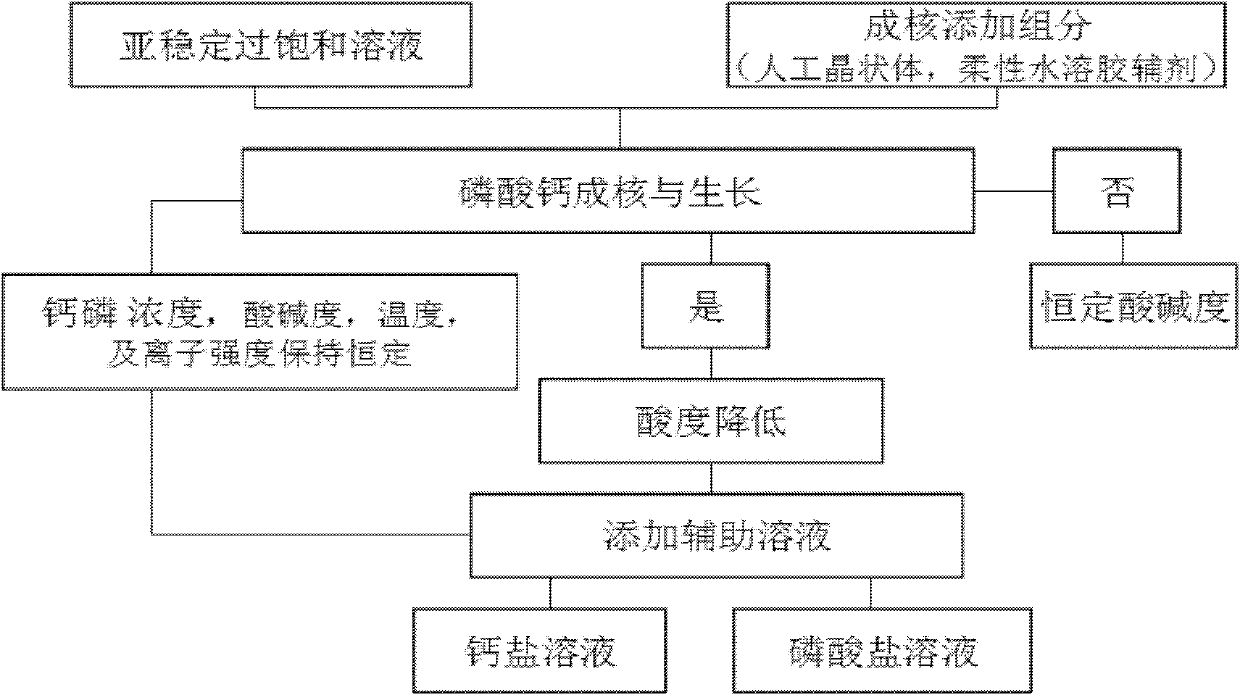
Patents
Literature
38 results about "Disodium Hydrogen Citrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Disodium citrate, more properly, disodium hydrogen citrate, is an acid salt of citric acid with the chemical formula Na 2 C 6 H 6 O 7. It is used as an antioxidant in food and to improve the effects of other antioxidants. It is also used as an acidity regulator and sequestrant.
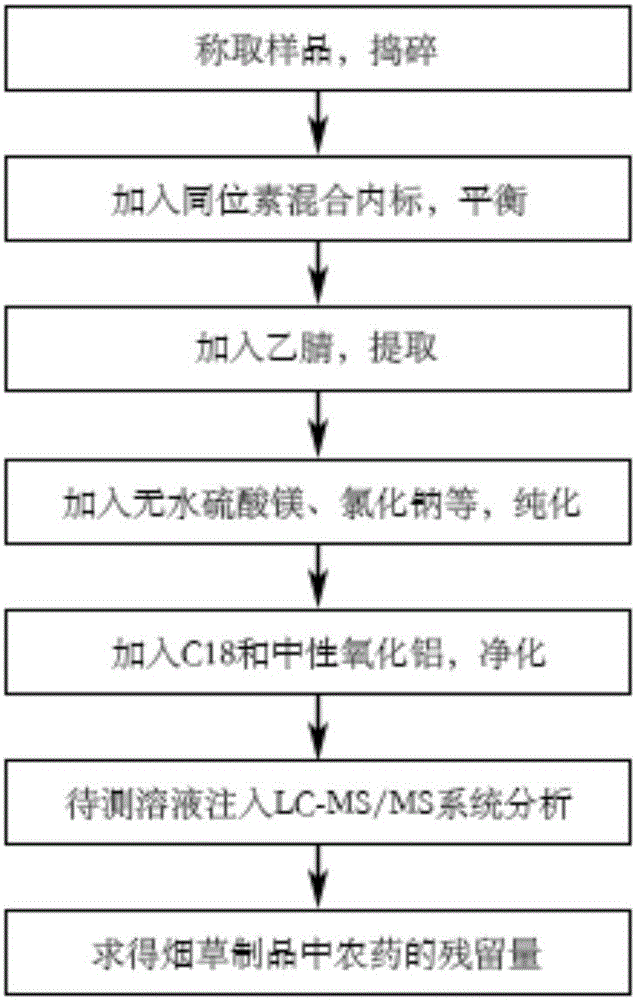
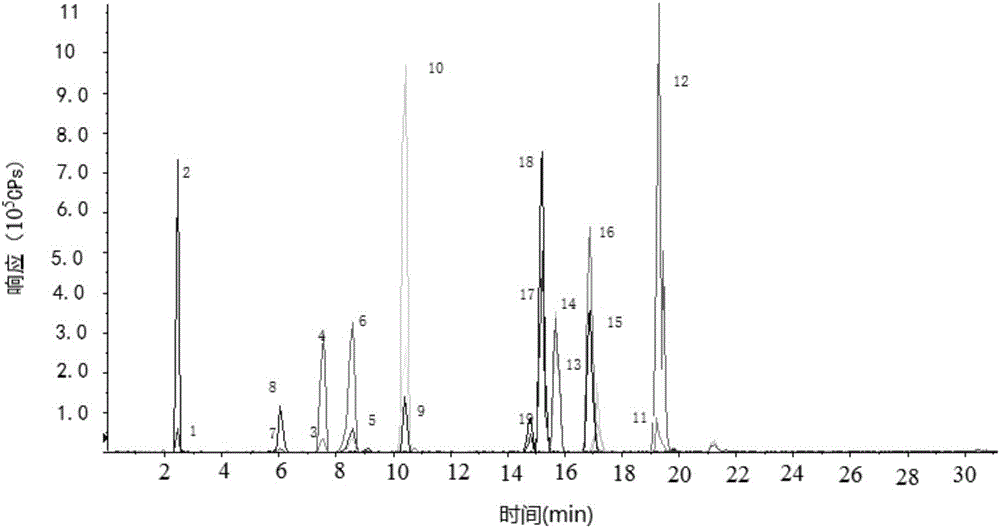
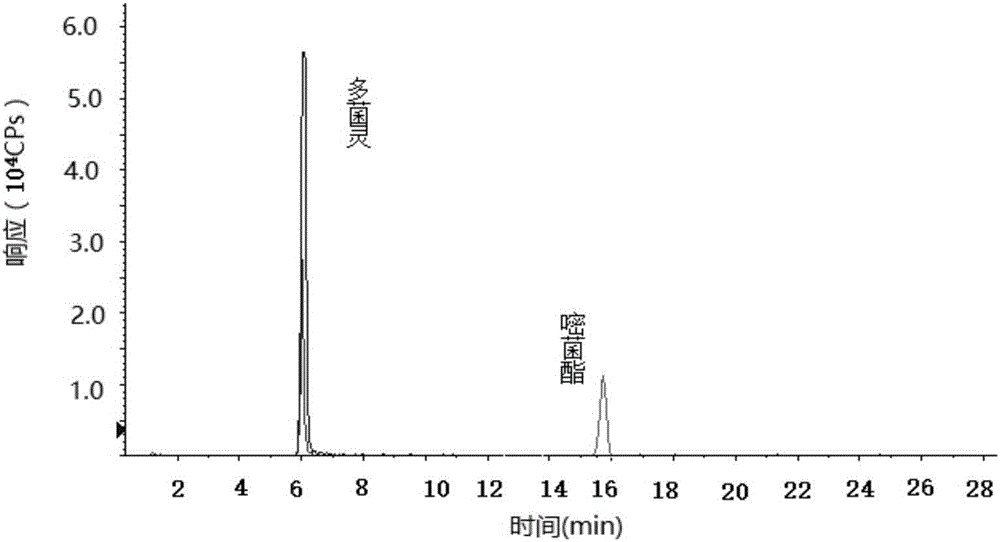
Determination method of pesticide residual quantity in tobacco and tobacco products
ActiveCN106324123AFast handlingImprove removal efficiencyComponent separationPesticide residueTobacco product
The invention discloses a determination method of the pesticide residual quantity in tobacco and tobacco products and belongs to the field of pesticide residue detection. According to the method, sample pretreatment is easy and fast to operate, the removal efficiency of impurities and interferents is high, and treatment time can be greatly shortened. After being extracted with acetonitrile, a sample is purified with anhydrous magnesium sulfate, sodium chloride, sodium citrate and disodium hydrogen citrate first and then purified with C18 and neutral aluminum oxide, in this way, pigments in the tobacco and other co-extracts (such as fatty acid and chlorophyll) of tobacco plant tissue can be effectively removed, and losses, caused by sample concentration, purification, derivation and other operation, of to-be-tested pesticide components are avoided, The determination method has the advantages that a blank matrix standard matching method cannot be adopted for quantitative analysis because mouth-held cigarettes have many varieties and matrixes are different, and an isotope internal standard dilution method can lower the influence of the matrix effect on the quantitative result; operation is easy and rapid, detection sensitivity is high, and repeatability is good.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
Medicinal composition containing thymopentin compound
ActiveCN102988954AImprove stabilityImprove tolerancePowder deliveryPeptide/protein ingredientsEthylene diamineVitamin C
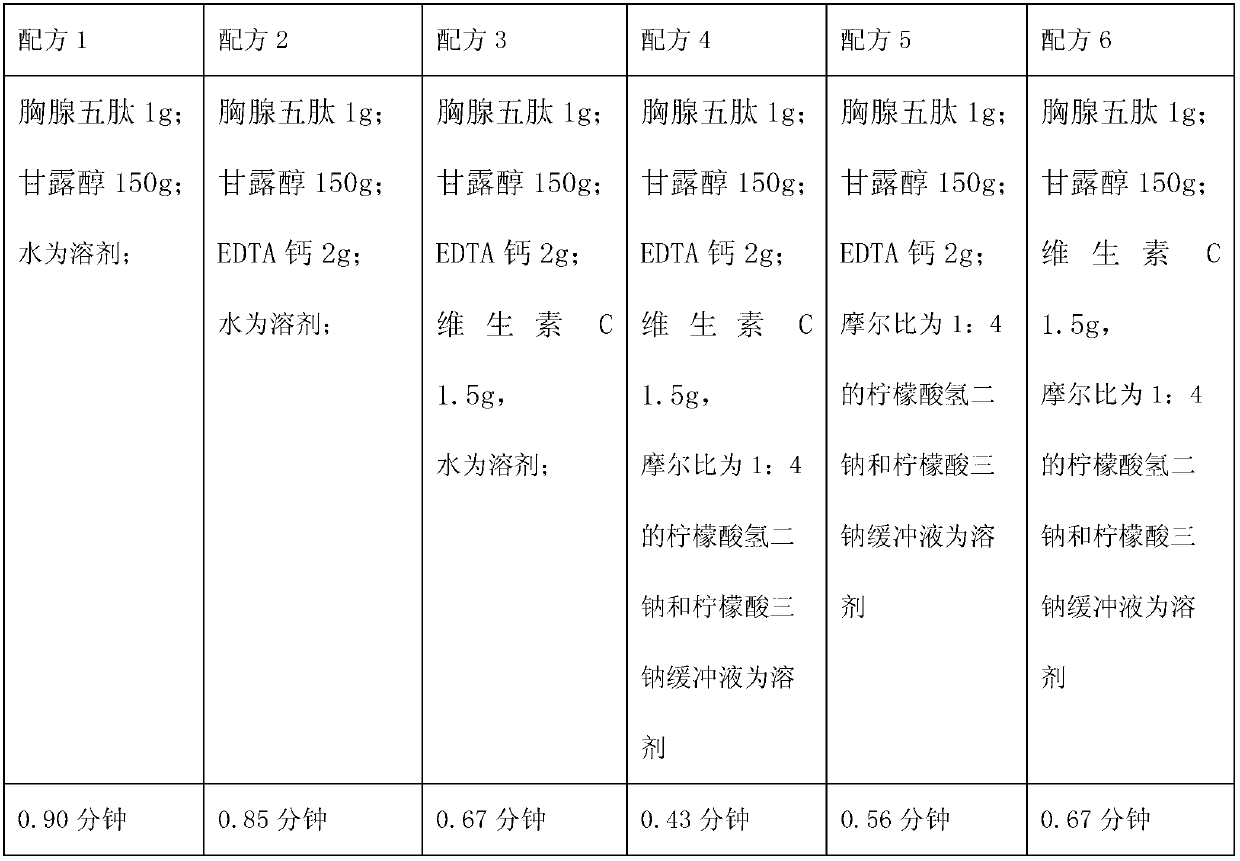
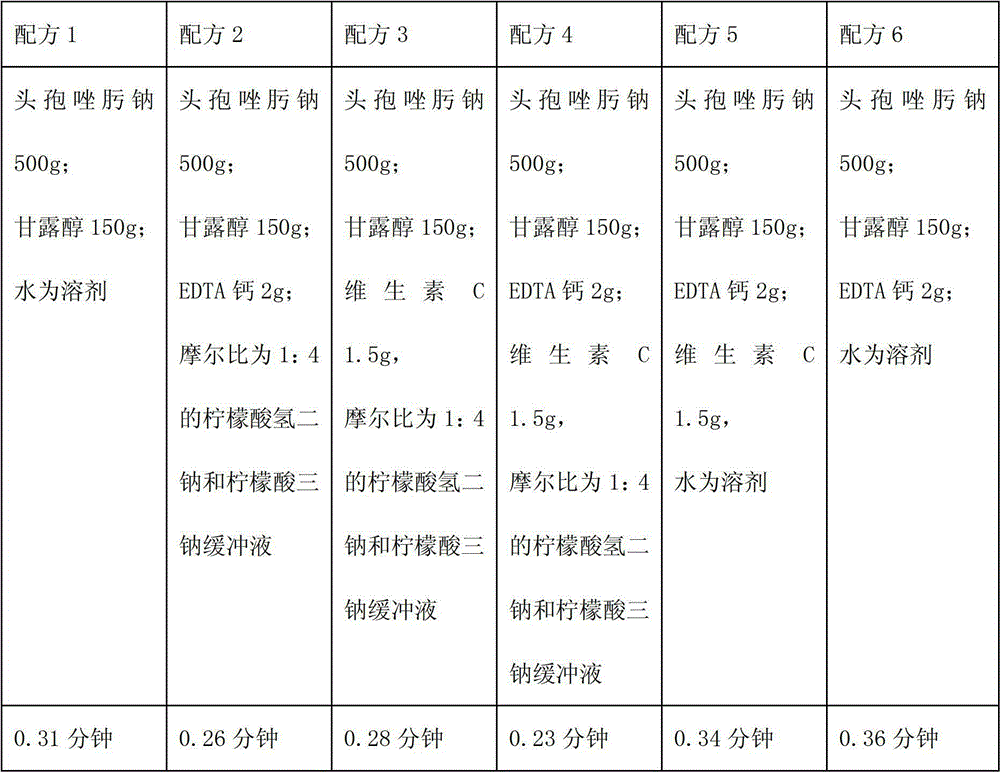
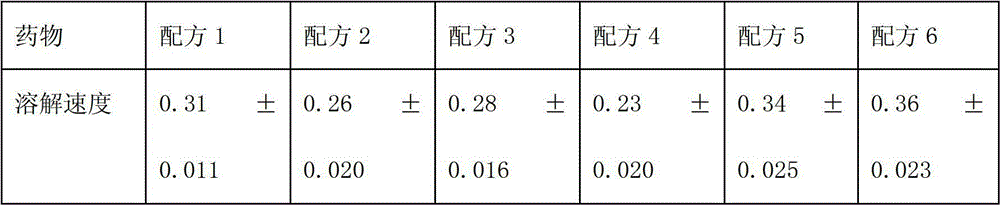
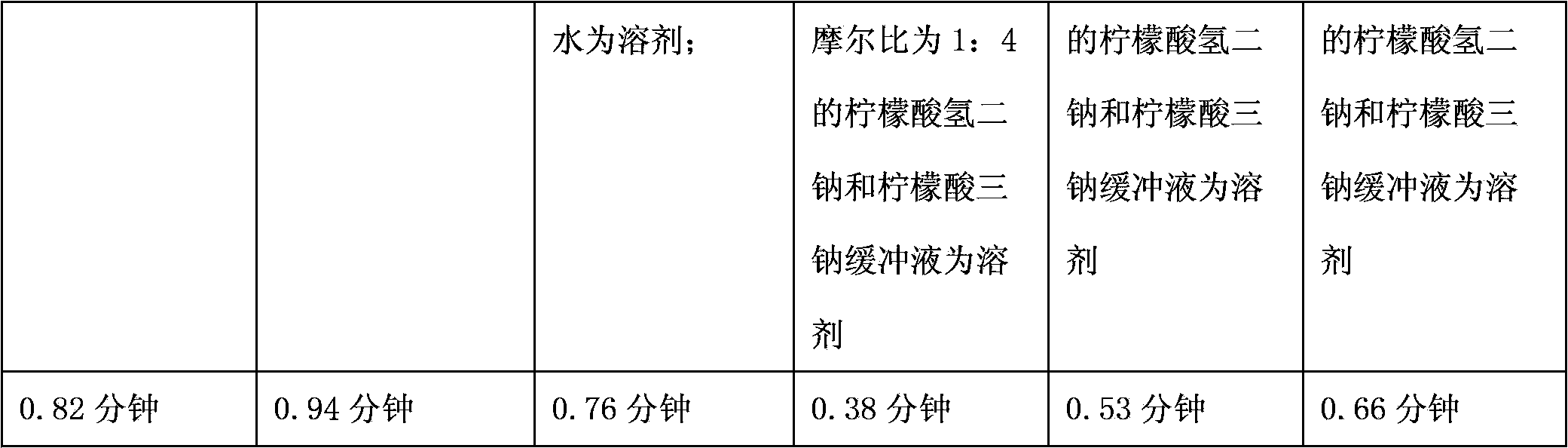
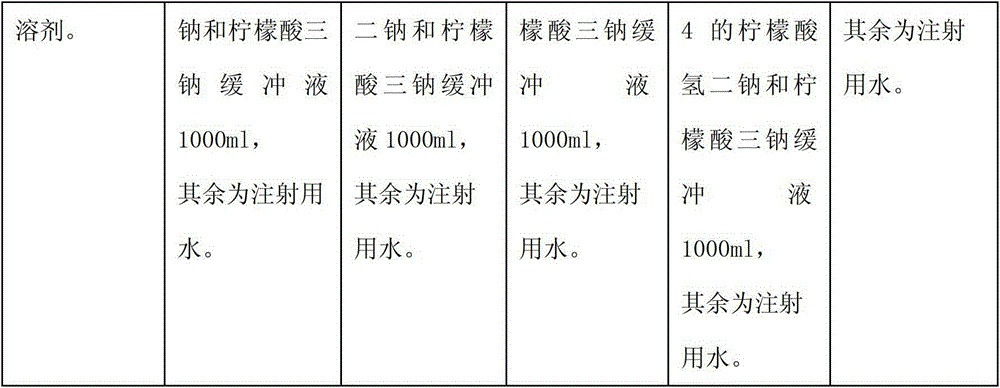
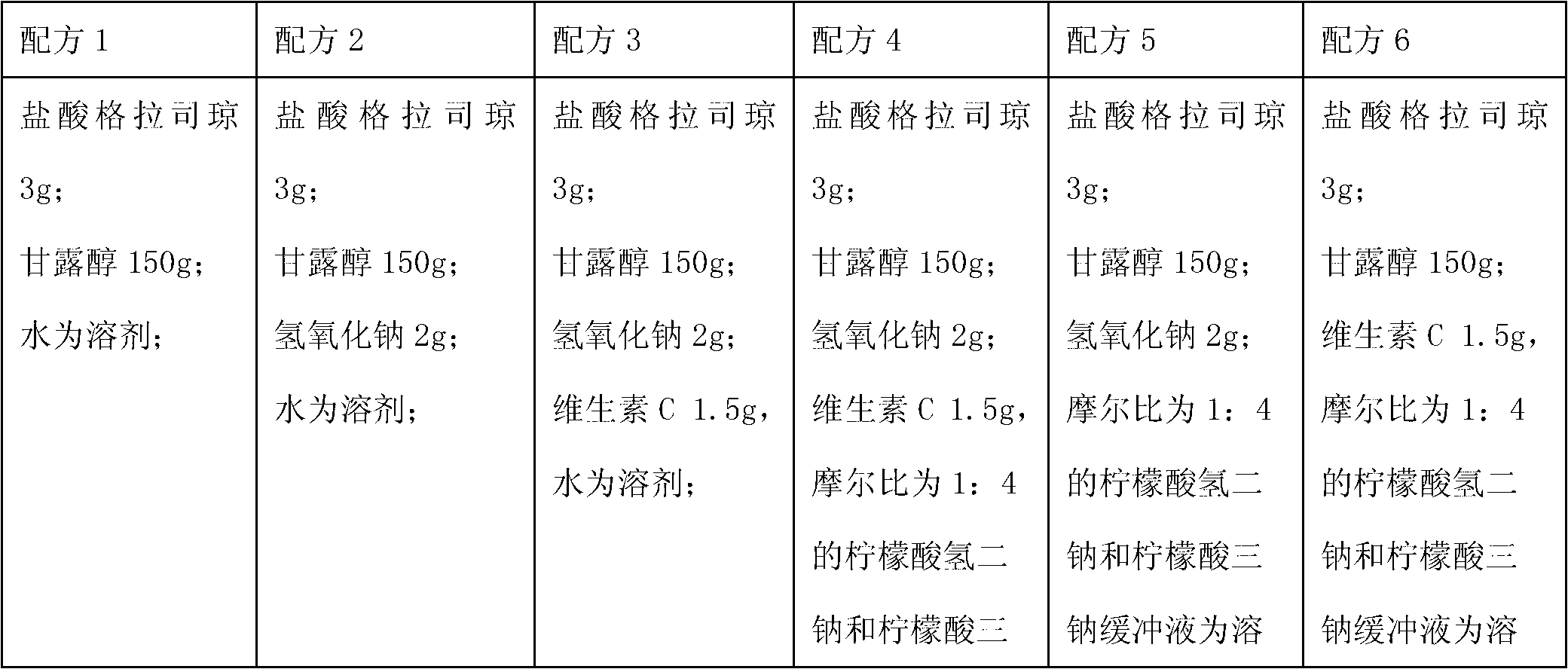
The invention relates to a medicinal composition containing a thymopentin compound, and in particular relates to an injection as well as a formula, application and a preparation method thereof. Each 1,000 injections are prepared from the following components: 0.5 to 2g of thymopentin, 50 to 200g of mannitol, 1 to 3g of ethylene diamine tetraacetic acid (EDTA) calcium, 1 to 2g of vitamin C, 1,000ml of buffer solution of disodium hydrogen citrate and trisodium citrate at a mole ratio of 1: 4, and the balance of injection water to the volume of 2,000ml.
Owner:姚云
Method and composition for extracting and purifying hymexazol pesticide residues in tobacco
The invention belongs to the field of detection of pesticide residues and relates to a method for extracting and purifying hymexazol pesticide residues in a tobacco sample, a method for detecting hymexazol pesticide residues and a composition or a kit for extracting and purifying hymexazol or detecting hymexazol. Specifically, the method for extracting and purifying the hymexazol pesticide residues in the tobacco sample comprises the following steps: (1) soaking the tobacco sample in an alkaline solution, adding ethyl acetate and oscillating to obtain a primary extraction solution; and (2) mixing the obtained primary extraction solution with any one of reagent groups A-C, standing the mixture, and extracting a supernatant solution 1, wherein the reagent group A comprises magnesium sulfate and sodium chloride, the reagent group B comprises magnesium sulfate, sodium chloride, sodium citrate and disodium hydrogen citrate sesquihydrate, and the reagent group C comprises magnesium sulfate, sodium acetate trihydrate and acetic acid. The method for extracting and purifying the hymexazol pesticide residues in the tobacco sample is high in hymexazol extraction rate, small in pollution and low in cost.
Owner:CHINA TOBACCO FUJIAN IND
Cefmetazole-containing pharmaceutical composition
ActiveCN102727451ASmall fluctuation rangeGuaranteed stabilityAntibacterial agentsOrganic active ingredientsHydrogenVitamin C
The invention relates to a novel pharmaceutic preparation composition, especially to an injection preparation of cefmetazole. The injection preparation comprises the following components: cefmetazole, mannitol, EDTA-Ca, vitamin C, sodium hydrogen citrate, a trisodium citrate buffer solution and water for injection.
Owner:哈药集团股份有限公司 +1
Ceftizoxime sodium-containing pharmaceutical composition
ActiveCN102716075AImprove stabilityImprove toleranceAntibacterial agentsOrganic active ingredientsEthylene diamineVitamin C
The invention relates to a novel pharmaceutical preparation composition, in particular to a ceftizoxime sodium-containing preparation for injection and a preparation method for the ceftizoxime sodium-containing preparation. The ceftizoxime sodium-containing preparation for injection comprises the following components: ceftizoxime sodium, mannitol, EDTA (Ethylene Diamine Tetraacetic Acid) calcium,vitamin C, sodium hydrogen citrate, 2000ml of trisodium citrate buffer solution and water for injection.
Owner:哈药集团股份有限公司 +1
Etchant composition for a single molybdenum film
ActiveCN102648269ASo as not to damageSimplify the etch processSurface treatment compositionsCITRATE ESTERMonosodium citrate
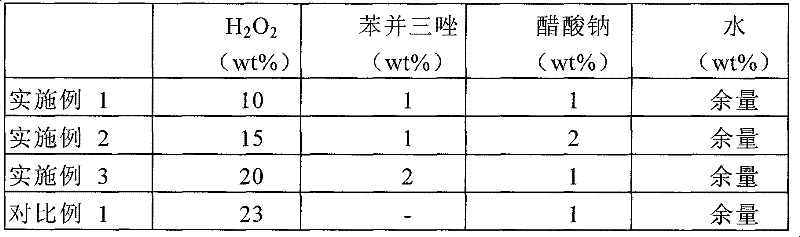
The present invention relates to an etchant composition for a single molybdenum film, comprising: 5 to 25 wt % of H2O2; 0.5 to 3 wt % of a cyclic amine compound; 0.5 to 5 wt % of an additive containing one or two or more selected from among a group consisting of sodium dihydrogen citrate, disodium hydrogen citrate, disodium hydrogen phosphate, trisodium citrate and acetate salts; with the remainder being water.
Owner:DONGWOO FINE CHEM CO LTD
Zinc-nickel alloy trivalent chromium black passivator and preparation process thereof
InactiveCN109267056AImprove stabilityGood colorMetallic material coating processesChromium CompoundsAcetic acid
The invention provides a zinc-nickel alloy trivalent chromium black passivator and a preparation process thereof. The passivator comprises an agent A, an agent B and water, wherein the agent A comprises a trivalent chromium compound, polyoxyethylene, disodium hydrogen citrate and water, and the agent B comprises glyceryl ether, glacial acetic acid and water; the trivalent chromium compound, polyoxyethylene and water are mixed to obtain a trivalent chromium compound solution, and a disodium hydrogen citrate solution is dropwise added to the trivalent chromium compound solution to obtain the agent A; the glyceryl ether, glacial acetic acid and water are mixed to obtain the agent B; the agent A and the agent B are mixed to obtain the zinc-nickel alloy trivalent chromium black passivator. Theblack passivator is more excellent in stability, a generated passivation layer is better in appearance and full in color, and the corrosion resistance is improved to a certain extent.
Owner:武汉风帆电化科技股份有限公司
Artificial lens implantation flexible hydrosol auxiliary agent and preparation method thereof
The invention discloses an artificial lens implantation flexible hydrosol auxiliary agent, which comprises the following components in percentage by mass: 0 to 5.0 percent of disodium hydrogen citrate, 0 to 2.5 percent of monosodium hydrogen citrate, 1.0 to 20 percent of carboxyl-propyl methylcellulose, 0.05 to 5.0 percent of sodium chondroitin sulfate, 0.1 to 10 percent of potassium chloride, 0.05 to 10 percent of magnesium chloride, 0.1 to 2.5 percent of sodium acetate, 0.1 to 1.0 percent of sodium chloride, and 44 to 99 percent of sterile double-distilled water. The artificial lens implantation flexible hydrosol auxiliary agent has excellent flow properties, can effectively reduce organism exclusion and prolong the service life of a lens, and particularly can better slow down postoperative lens turbidity, so that the artificial lens implantation flexible hydrosol auxiliary agent is expected to fundamentally reduce pains of patients and save expensive cost caused by repeated operations, and has a great expectancy of social benefits and economic benefits.
Owner:高家旗
Medicinal composition containing meglumine cyclic adenosine monophosphate compound
ActiveCN102988305BImprove stabilityImprove tolerancePowder deliveryOrganic active ingredientsEthylene diamineVitamin C
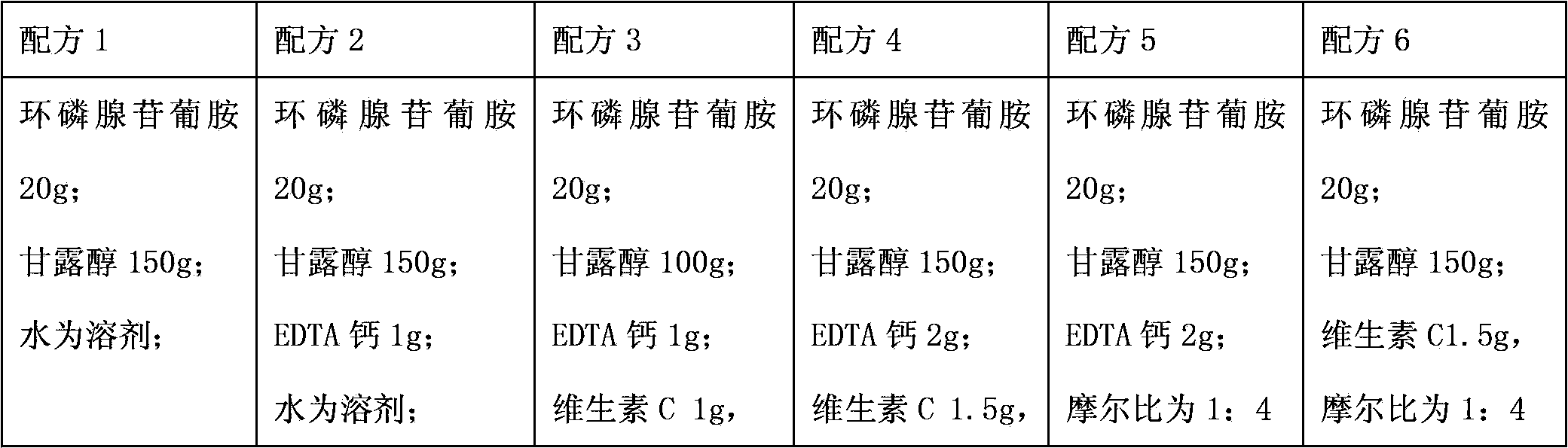
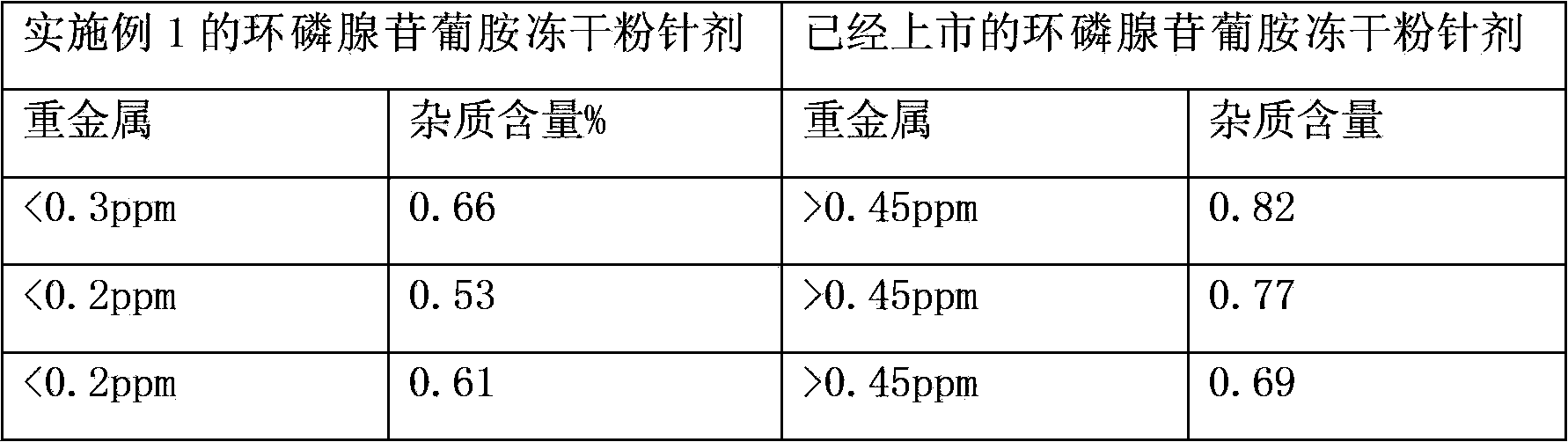
The invention relates to a medicinal composition containing a meglumine cyclic adenosine monophosphate compound, and in particular relates to freeze-dried injection of meglumine cyclic adenosine monophosphate and a preparation method thereof. Each 1,000 injections are prepared from the following components: 20g of meglumine cyclic adenosine monophosphate, 100 to 200g of mannitol, 1 to 3g of ethylene diamine tetraacetic acid (EDTA) calcium, 1 to 2g of vitamin C, and 2,000ml of a buffer solution of disodium hydrogen citrate and trisodium citrate at a mole ratio of 1: 4.
Owner:姚云
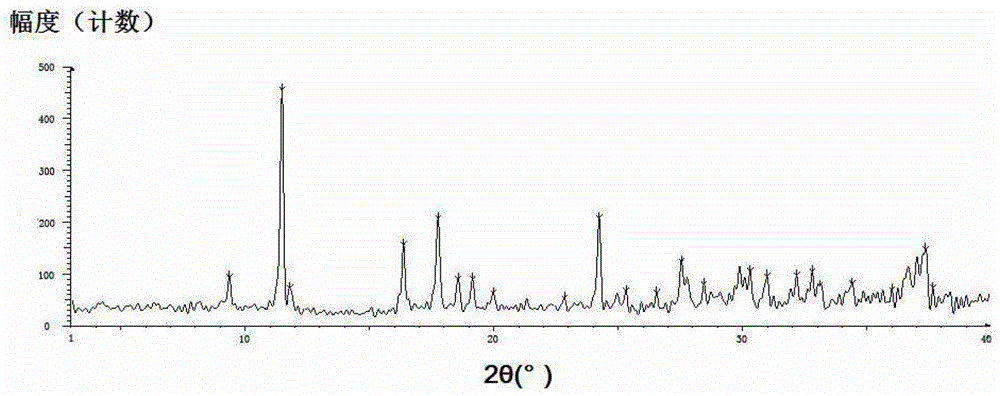
Polymorphism of desloratadine disodium hydrogen citrate complex salt
ActiveCN103242292AIncrease humidityImprove solubilityCarboxylic compound separation/purificationX-raySolvent
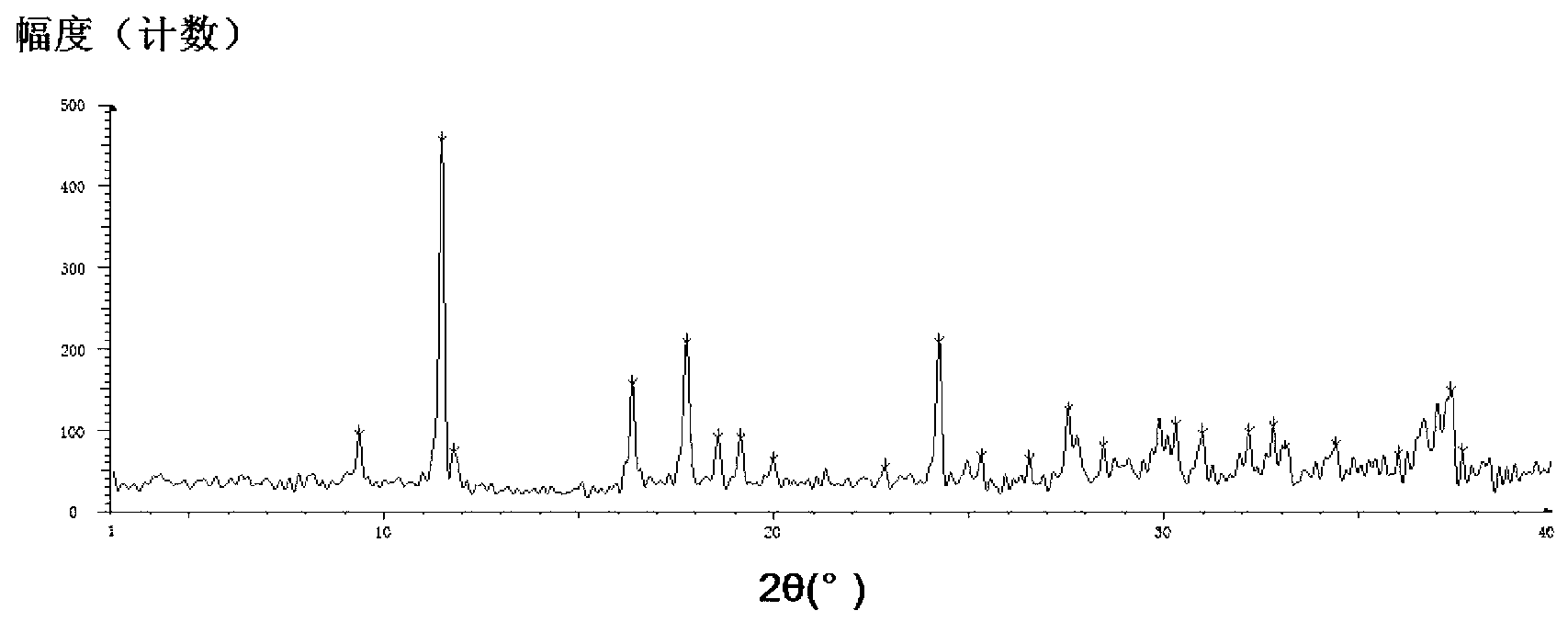
The invention belongs to the field of medicinal chemistry and specifically relates to a polymorphic and amorphous state of a desloratadine disodium hydrogen citrate complex salt and a preparation method thereof. The polymorphic and amorphous state is characterized in that an X-ray powder diffraction pattern of polymorphism has characteristic peaks in the vicinity of 2 theta angles of 11.45 degrees, 16.36 degrees, 17.74 degrees, 18.55 degrees, 19.13 degrees, 24.23 degrees, 27.54 degrees, 30.99 degrees, 32.82 degrees and 37.39 degrees. The preparation method of the polymorphic and amorphous state of the desloratadine disodium hydrogen citrate complex salt, disclosed by the invention, has the benefits of simplicity in operation, small using quantity of solvents, no toxicity and low production cost, and further has obvious advantages in the aspect of industrialization.
Owner:HEFEI AMVITE PHARM CO LTD +1
Measurement method for the cyfluthrin pesticide residue in tea
InactiveCN105424863ASimple extraction methodGood effectComponent separationPesticide residueRelative standard deviation
The invention relates to a measurement method for the cyfluthrin pesticide residue in tea. The method comprises the following steps: firstly, sample preparation is carried out, namely, tea is dried, ground and sieved; secondly, cyfluthrin crude extraction is carried out, namely, the tea powder is placed in a centrifuge tube, water is added for infiltration, petroleum ether and a mixed internal standard operating fluid are added, the mixture is shocked and mixed uniformly, anhydrous magnesium sulfate, sodium chloride, sodium citrate, sodium hydrogencitrate and methylbenzene are added, and the mixture is shocked uniformly and centrifuged; thirdly, cyfluthrin crude extract product purifying is carried out, namely, the supernate is taken and placed in a centrifuge tube, anhydrous magnesium sulfate and N-propylethylenediamine bonding solid adsorbents are added, the mixture is shocked uniformly and centrifuged; fourthly, cyfluthrin extraction is carried out, namely, the supernate is taken, extraction, condensation and filtering are carried out in a Florisil column by utilization of the toluene-isooctane mixed solution; fifthly, chromatographic detection is carried out. The extraction method is simple, the effects are good, and result integration processing is simple and accurate. The average recovery rate of the method is 93.21%-103.75%, the relative standard deviation is 3.28%-6.20%, the average recovery rate and the relative standard deviation meet requirements and the method is accurate and reliable.
Owner:周德志
Measurement method for the cypermethrin pesticide residue in tea
InactiveCN105424862ASimple extraction methodGood effectComponent separationCypermethrinPesticide residue
The invention relates to a measurement method for the cypermethrin pesticide residue in tea. The method comprises the following steps: firstly, sample preparation is carried out, namely, tea is dried, ground and sieved; secondly, cypermethrin crude extraction is carried out, namely, the tea powder is placed in a centrifuge tube, water is added for infiltration, petroleum ether and a mixed internal standard operating fluid are added, the mixture is shocked and mixed uniformly, anhydrous magnesium sulfate, sodium chloride, sodium citrate, sodium hydrogencitrate and methylbenzene are added, and the mixture is shocked uniformly and centrifuged; thirdly, cypermethrin crude extract product purifying is carried out, namely, the supernate is taken and placed in a centrifuge tube, anhydrous magnesium sulfate and N-propylethylenediamine bonding solid adsorbents are added, the mixture is shocked uniformly and centrifuged; fourthly, cypermethrin extraction is carried out, namely, the supernate is taken, extraction, condensation and filtering are carried out in a Florisil column by utilization of the toluene-isooctane mixed solution; fifthly, chromatographic detection is carried out. The extraction method is simple, the effects are good, and result integration processing is simple and accurate. The average recovery rate of the method is 93.21%-103.75%, the relative standard deviation is 3.28%-6.20%, the average recovery rate and the relative standard deviation meet requirements and the method is accurate and reliable.
Owner:周德志
Determination method of residual quantity of flucythrinate pesticide in tea
InactiveCN105388235ASimple extraction methodGood effectComponent separationEthylenediamineWorking fluid
The invention relates to a determination method of residual quantity of flucythrinate pesticide in tea. The determination method comprises the following steps: (1) sample preparation: drying tea, grinding and screening; (2) flucythrinate coarse extraction: placing tea powder into a centrifuge tube, wetting by adding water, adding petroleum ether and mixed interior label working fluid, vibrating and uniformly mixing, adding anhydrous magnesium sulfate, sodium chloride, sodium citrate, sodium hydrogen citrate and methylbenzene, uniformly vibrating, and centrifuging; (3) flucythrinate coarse extract purification: placing liquid supernatant into the centrifuge tube, adding anhydrous magnesium sulfate and a N-propyl ethylenediamine bonding solid phase adsorbing agent, uniformly vibrating, and centrifuging; (4) flucythrinate extraction: taking supernatant, and extracting, concentrating and filtering on a Florisil column by using a methylbenzene-isooctane mixed solution; (5) chromatographic detection. The extraction method provided by the invention is simple and good in effect, and the result integral processing is simpler and more accurate. The average recovery rate of the method is 93.21 percent to 103.75 percent, the relative standard deviation is 3.28 percent to 6.20 percent, the requirements are met, and accuracy and reliability are realized.
Owner:周德志
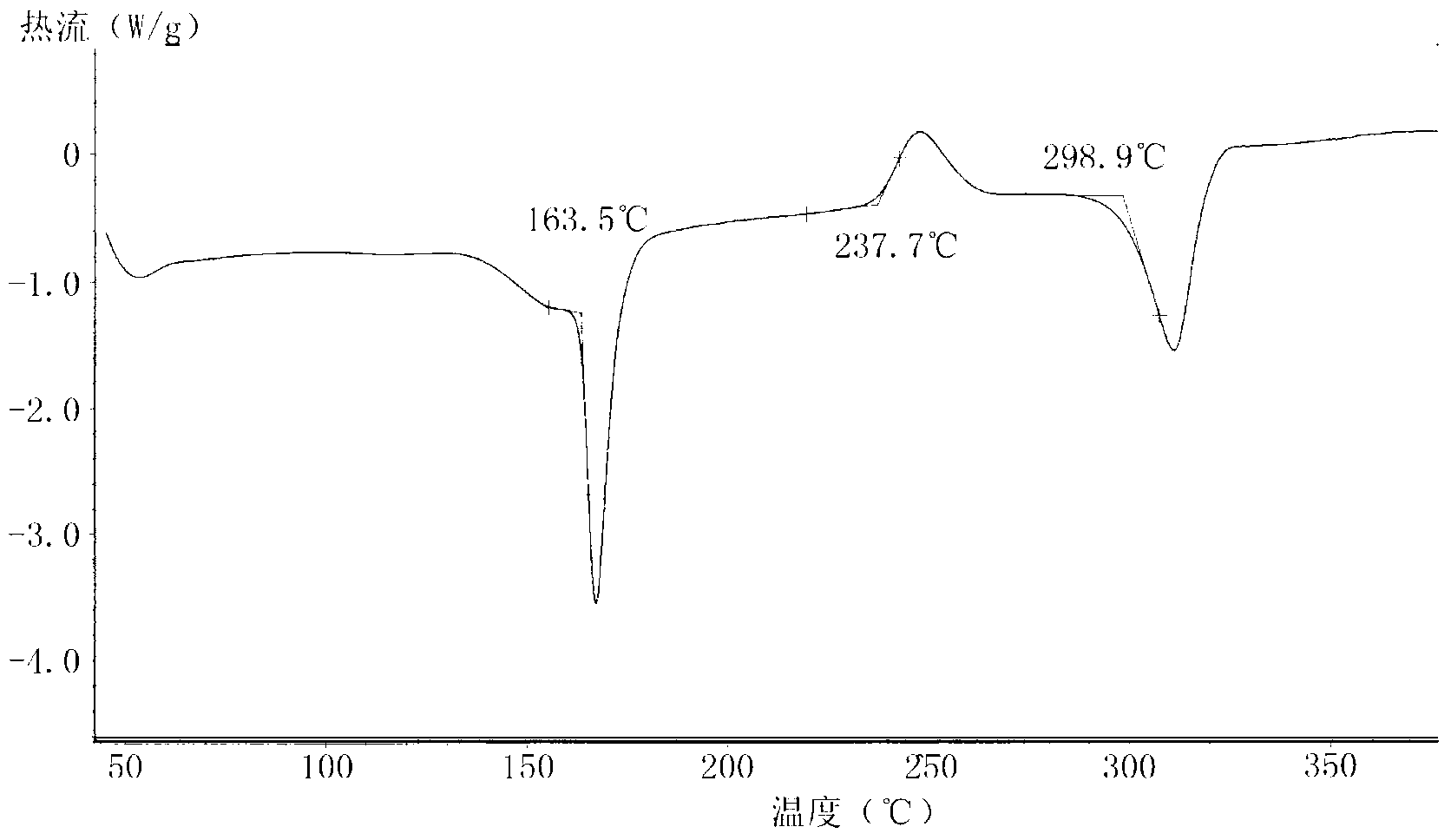
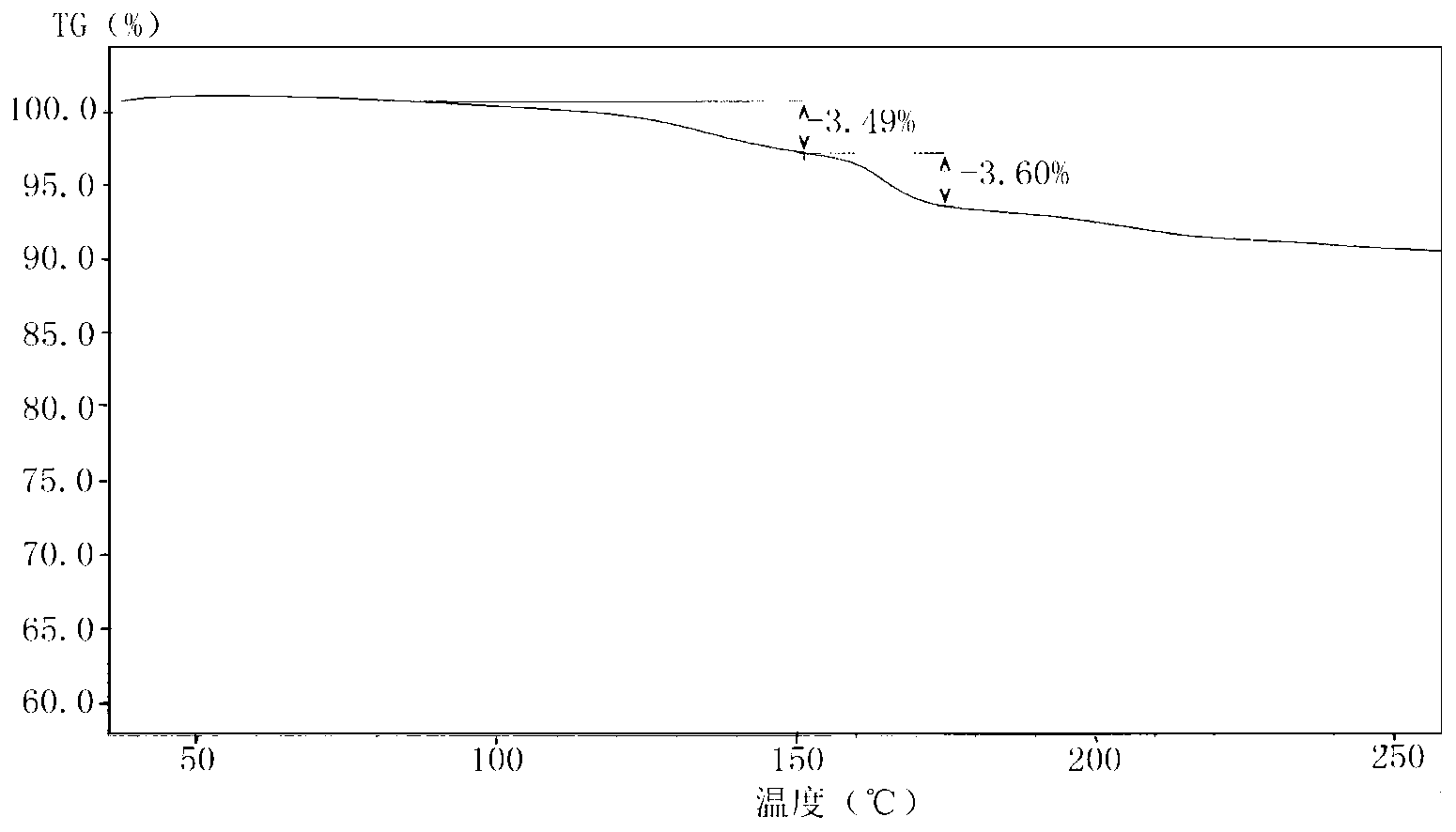
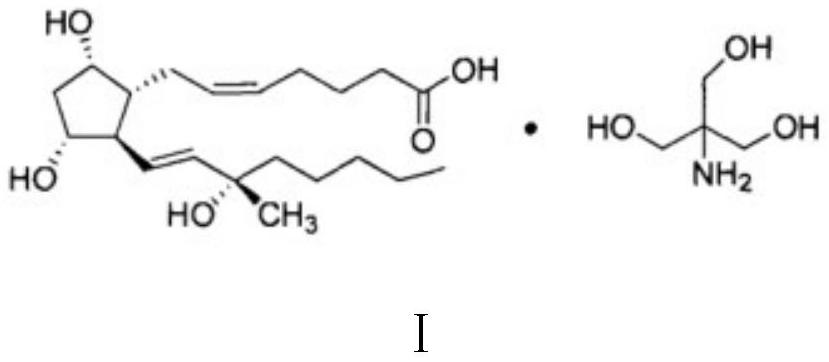
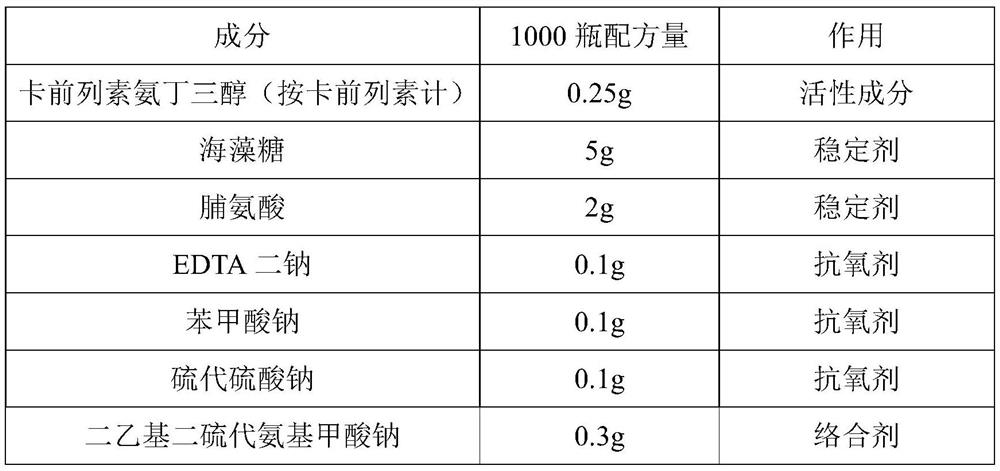
Carboprost tromethamine injection and preparation method thereof
ActiveCN112057417AImprove quality stabilityGrowth inhibitionOrganic active ingredientsPharmaceutical delivery mechanismThio-Ethyl group
The invention relates to a carboprost tromethamine injection and a preparation method thereof. The injection contains carboprost tromethamine, a stabilizer, an antioxidant, a complexing agent and a buffer solution. The injection is characterized in that the stabilizer is trehalose and proline, the antioxidant is disodium EDTA, sodium benzoate and sodium thiosulfate, the complexing agent is sodiumdiethyldithiocarbamate, and the buffer solution is disodium hydrogen citrate and trisodium citrate buffer solution. Meanwhile, the invention discloses a corresponding preparation method. Compared withthe prior art, the preparation method has the advantages that the stability of the carboprost tromethamine is remarkably improved, particularly, two main impurities including a chiral isomer and a 5,6-trans isomer on 15-site carbon can be remarkably reduced, the product quality is improved, the toxic and side effects are reduced, and the preparation method is simple, low in cost and suitable forindustrial large-scale production.
Owner:CHANGZHOU NO 4 PHARMA FACTORY +1
Method for determining tefluthrin pesticide residue in tea
InactiveCN105548399ASimple extraction methodGood effectComponent separationPesticide residueRelative standard deviation
The invention relates to a method for determining tefluthrin pesticide residue in tea. The method comprises the following steps: (1) preparing a sample: drying tea, grinding and sieving; (2) roughly extracting tefluthrin: putting the tea powder in a centrifugal tube, adding water for wetting, adding petroleum ether and mixing an internal standard working solution, uniformly shaking, adding anhydrous magnesium sulfate, sodium chloride, sodium citrate, sodium hydrogen citrate and methylbenzene, uniformly shaking and centrifuging; (3) purifying crude extract of tefluthrin: taking liquid supernatant and putting into the centrifugal tube, adding anhydrous magnesium sulfate and N-propyl quadrol bonding solid phase adsorbent, uniformly shaking and centrifuging; (4) extracting tefluthrin: taking the liquid supernatant and taking a methylbenzene-isooctane mixed solution for extracting, concentrating and filtering on a Florisil column; and (5) performing chromatography detection. The extraction method provided by the invention is simple and good in effect; the result integral treatment is more convenient and accurate; and the method provided by the invention has the average recovery rate of 93.21%-103.75% and the relative standard deviation of 3.28%-6.20%, thereby meeting requirements and being accurate and reliable.
Owner:周德志
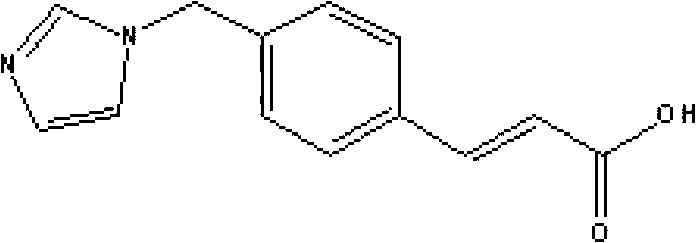
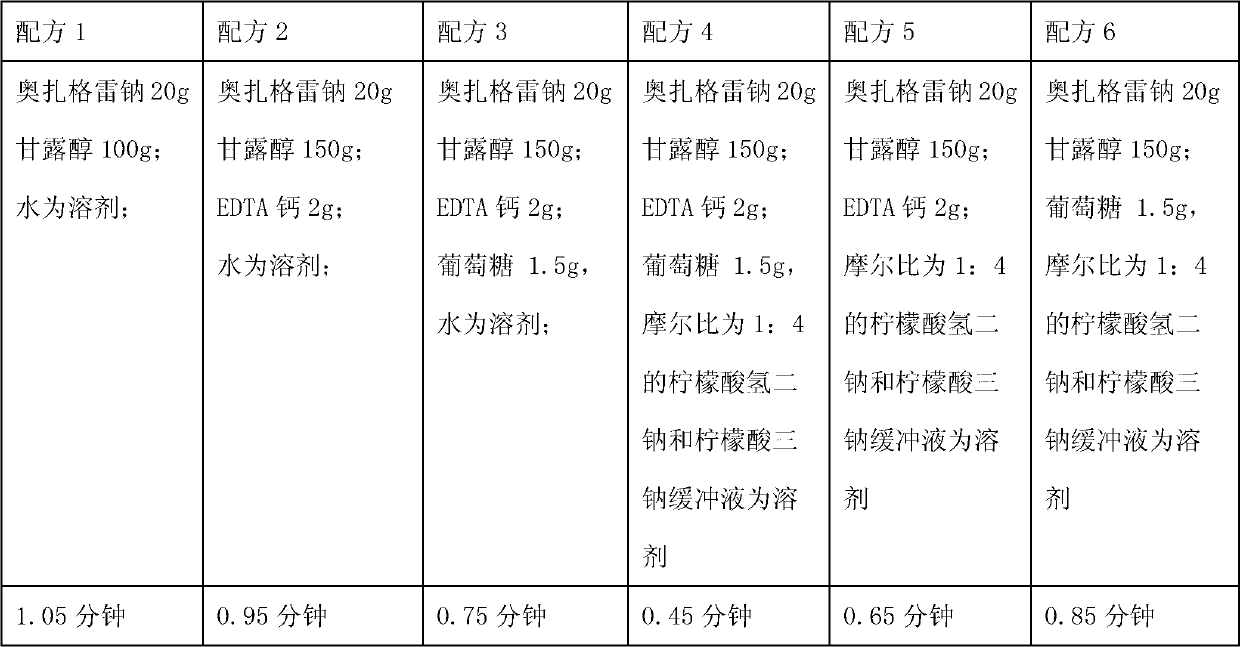
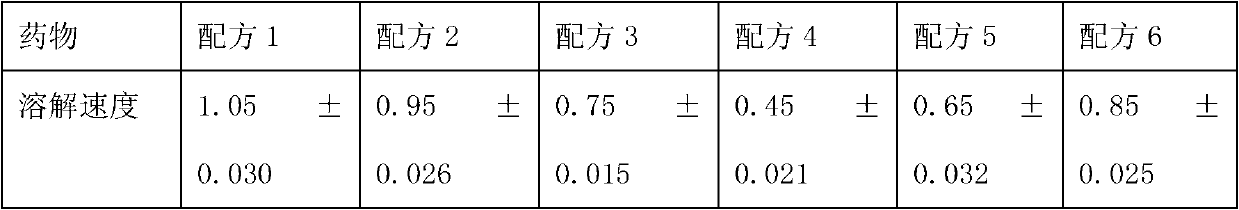
Medicinal composition containing sodium ozagrel compound
ActiveCN102988306AImprove stabilityImprove tolerancePowder deliveryOrganic active ingredientsEthylene diamineFreeze-drying
The invention relates to a medicinal composition containing sodium ozagrel, and the medicinal composition is a freeze-dried injection. Each 1,000 injections are prepared from the following components: 20g of sodium ozagrel, 100 to 200g of mannitol, 1 to 3g of ethylene diamine tetraacetic acid (EDTA) calcium, 1 to 2g of glucose, and 2,000ml of buffer solution of disodium hydrogen citrate and trisodium citrate at a mole ratio of 1: 4, wherein the pH value of the injection is 7.0.
Owner:姚云
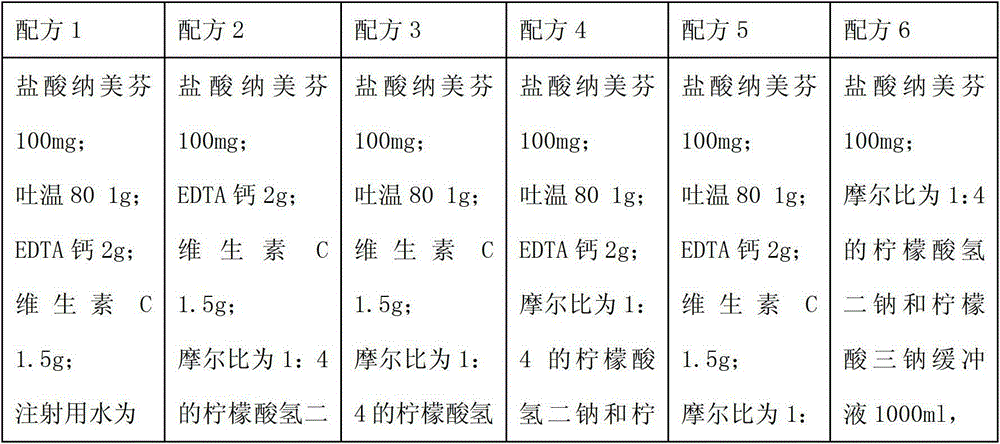
Pharmaceutical composition containing nalmefene hydrochloride compound
ActiveCN103040733BImprove stabilityImprove toleranceOrganic active ingredientsNervous disorderEthylene diamineVitamin C
The invention relates to a pharmaceutical composition containing a nalmefene hydrochloride compound. For every 1000 injections, the pharmaceutical composition comprises the following components: 100mg of nalmefene hydrochloride, 1-2g of Tween 80, 1-3g of EDTA (Ethylene Diamine Tetraacetic Acid) calcium, 1-2g of vitamin C, 1000ml of sodium hydrogencitrate and trisodium citrate buffer liquor with molar ratio of 1:4, and the balance of water for injection.
Owner:罗诚
Medicinal composition containing cefotiam hydrochloride
ActiveCN102716096ASmall fluctuation rangeGuaranteed stabilityAntibacterial agentsPowder deliveryVitamin CCITRATE ESTER
The invention relates to a new medical preparation composition, in particular to an injection preparation containing cefotiam hydrochloride, and application of the injection preparation. The composition comprises cefotiam hydrochloride, anhydrous sodium carbonate, mannitol, vitamin C, and a sodium hydrogen citrate and trisodium citrate buffering solution.
Owner:哈药集团股份有限公司 +1
Method for solubilizing and modifying yeast beta-glucan by ultrasonic-enzyme synergistic method
The invention discloses a method for solubilizing and modifying yeast beta-glucan by ultrasonic-enzyme synergistic method, which is characterized by comprising the following steps: respectively dissolving yeast beta-glucan and beta-glucanase in a disodium hydrogen citrate phosphate buffer solution to prepare a beta-glucanase solution and a yeast beta-glucan solution, adding the beta-glucanase solution into the yeast beta-glucan solution, and carrying out ultrasonic enzymolysis. The yeast beta-glucan product obtained by the method is colorless, tasteless and better in water solubility, and theoriginal molecular structure of the yeast beta-glucan can be maintained while the problem of lower solubility of the yeast beta-glucan in water is solved.
Owner:SHANGHAI INST OF TECH +1
Method for simultaneously determining multiple plasticizers and multiple pesticide residues in fruits
The invention discloses a method for simultaneously determining multiple plasticizers and multiple pesticide residues in fruits in the field of fruit detection, which comprises the following steps: S1: extraction: weighing 10g of fruit sample in a 50mL centrifuge tube, adding 10mL of acetonitrile, 4g of magnesium sulfate, 1g of sodium chloride, 1g of sodium citrate, 0.5g of disodium hydrogen citrate and one ceramic homoproton, covering the centrifuge tube, and after violently oscillating for 1 minute, centrifuging for 5 minutes at the speed of 4200 r / min; and S2, preparing a solution, namely transferring 1mL of 100 [mu]g / mL epoxy heptachloroB standard solution into a 10mL volumetric flask, fixing the volume to be an internal standard stock solution by using ethyl acetate, and diluting the internal standard stock solution to 5mg / L by using the ethyl acetate before use to obtain an internal standard solution. According to the invention, a sample is subjected to acetonitrile homogenate extraction, salting-out centrifugation, Quickers purification and nitrogen blowing, then an internal standard substance (epoxy heptachloroB) is added and redissolved with ethyl acetate, gas chromatography tandem mass spectrometry is used for determination, an internal standard method is used for quantification, and the method is simple, low in cost, safe, environmentally friendly and free of chemical reagent residues.
Owner:河北省农林科学院生物技术与食品科学研究所
Pharmaceutical composition containing granisetron hydrochloride compound
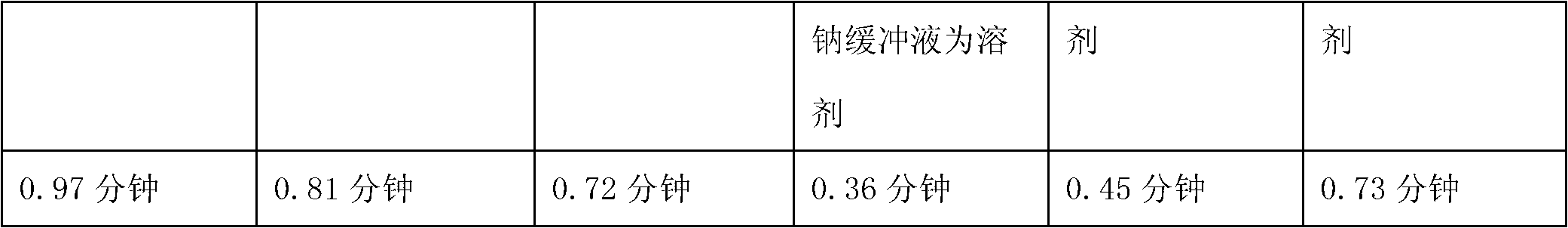
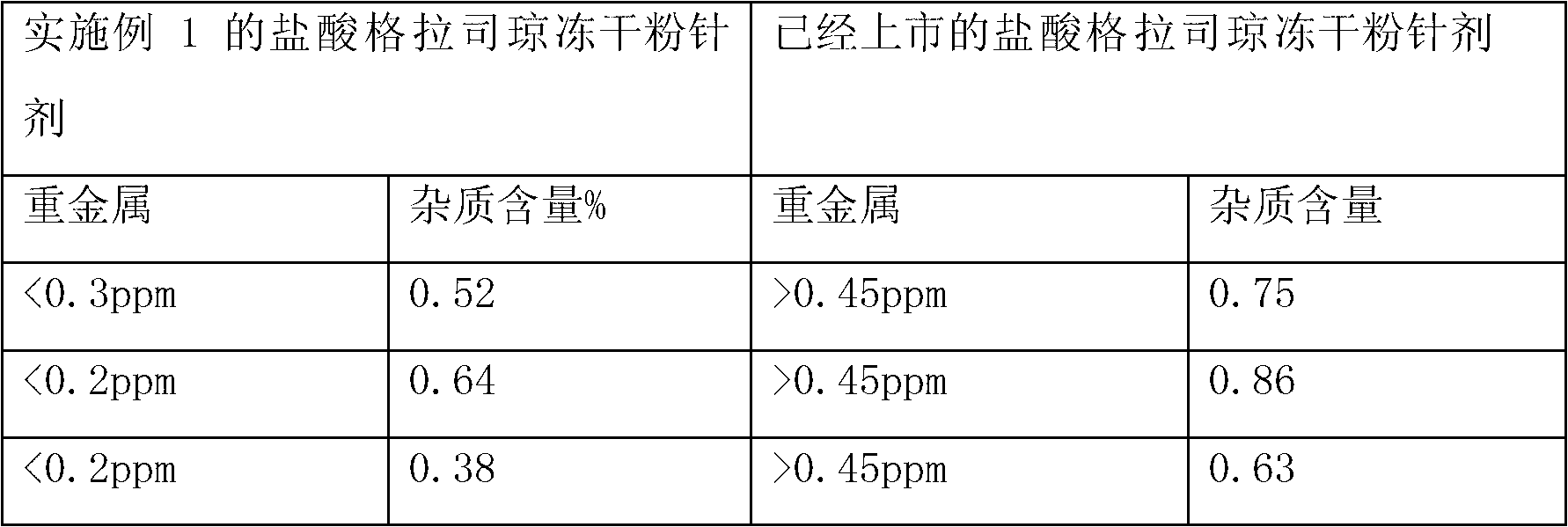
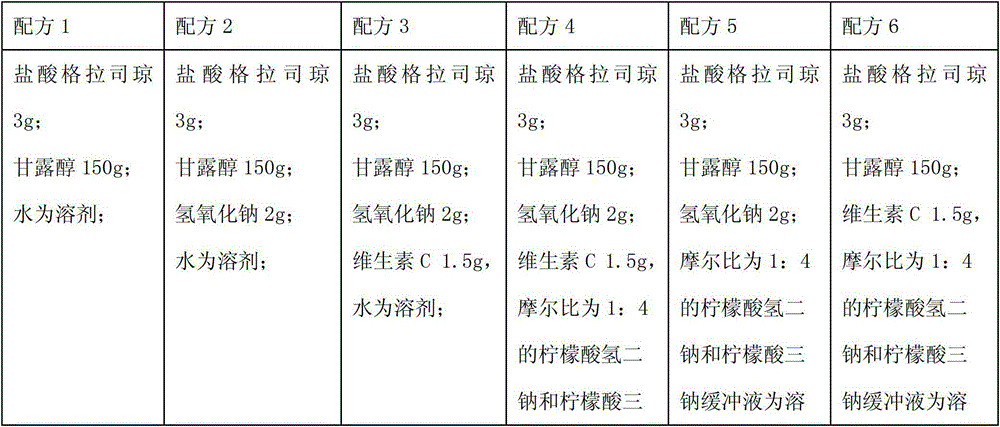
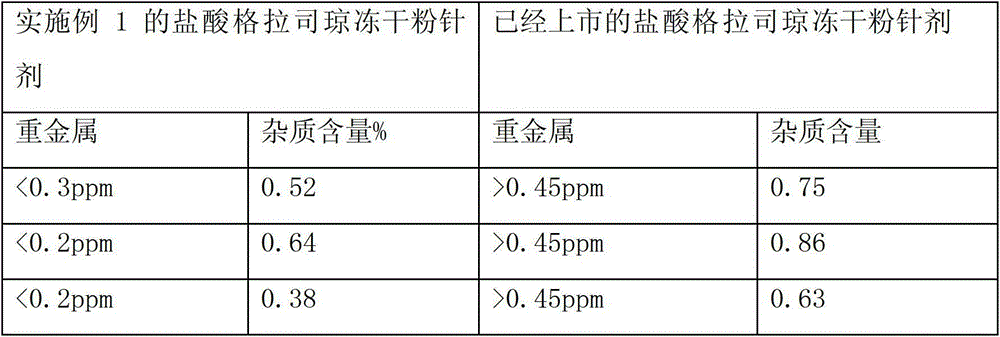
ActiveCN103040762AImprove stabilityImprove toleranceOrganic active ingredientsPowder deliveryVitamin CMANNITOL/SORBITOL
The invention relates to a pharmaceutical composition containing a granisetron hydrochloride compound, and in particular to a freeze-dried injection preparation of granisetron hydrochloride and a preparation method thereof. For every 1000 injections, the pharmaceutical composition comprises the following components: 3g of granisetron hydrochloride, 100-200g of mannitol, 1-3g of sodium hydroxide, 1-2g of vitamin C, and 2000ml of sodium hydrogencitrate and trisodium citrate buffer liquor with molar ratio of 1:4.
Owner:姚云
Pharmaceutical composition containing granisetron hydrochloride compound
ActiveCN103040762BImprove stabilityImprove toleranceOrganic active ingredientsPowder deliveryVitamin CFreeze-drying
The invention relates to a pharmaceutical composition containing a granisetron hydrochloride compound, and in particular to a freeze-dried injection preparation of granisetron hydrochloride and a preparation method thereof. For every 1000 injections, the pharmaceutical composition comprises the following components: 3g of granisetron hydrochloride, 100-200g of mannitol, 1-3g of sodium hydroxide, 1-2g of vitamin C, and 2000ml of sodium hydrogencitrate and trisodium citrate buffer liquor with molar ratio of 1:4.
Owner:姚云
Method for detecting residual amount of permethrin pesticide in tea leaves
InactiveCN105403643ASimple extraction methodGood effectComponent separationPesticide residueRelative standard deviation
The invention relates to a method for determining the residual quantity of a permethrin pesticide in tea leaves. The method comprises the following steps: 1, preparing a sample: drying the tea leaves, grinding the dried tea leaves, and sieving the ground tea leaves; 2, carrying out permethrin crude extraction: placing the powdered tea leaves in a centrifuge tube, adding water to infiltrate the powdered tea leaves, adding petroleum ether and a mixed internal standard operating fluid, vibrating until uniformity, adding anhydrous magnesium sulfate, sodium chloride, sodium citrate, disodium hydrogen citrate and toluene, vibrating until uniformity, and centrifuging; 3, purifying a permethrin crude extraction product: taking a supernatant obtained in step 2 to the centrifuge tube, adding anhydrous magnesium sulfate and an N-propylethylene diamine bonded solid phase adsorbent, vibrating until uniformity, and centrifuging; 4, carrying out permethrin extraction: taking a supernatant obtained in step 3, carrying out Florisil silica column extraction by using a toluene-isooctane mixed solution, concentrating, and filtering; and 5, carrying out chromatographic detection. The extraction method has the advantages of simplicity, good effect, and simple and accurate result integration treatment. The average recovery rate of the method is 93.21-103.75%, and the relative standard deviation is 3.28-6.20%, so the method accords with requirements, and is accurate and reliable.
Owner:周德志
Soft hydrosol auxiliary agent used for ophthalmology
ActiveCN104814977AReduce turbidityReduce dosageSenses disorderAlkali/alkaline-earth metal chloride active ingredientsSodium acetateHydrogen
The invention relates to medical preparations, and particularly relates to a soft hydrosol auxiliary agent used for ophthalmology, which comprises following components: 0.2-0.5% of disodium hydrogen citrate, 1-1.5% of monosodium hydrogen citrate, 5-8% of hydroxypropyl methylcellulose, 0.5-1% of sodium chondroitin sulfate, 0.5-2% of potassium chloride, 0.3-0.8% of magnesium chloride, 0.3-1.5% of sodium acetate, 1.3-2.5% of sodium chloride, and 85-90% of sterilized double-distilled water and also may comprises 1-3% of sodium hyaluronate. The auxiliary agent, on the premise of ensuring performance, reducing the risk of nucleation and deposition and alleviating lens opacity, is significantly reduced in use amounts of the disodium hydrogen citrate and the magnesium chloride, thereby reducing stimulation on eyes of patients and avoiding complications of itch, weeping and red swelling in eyes. In addition, the preparation is increased in bionic level. The preparation is significantly increased in stability, is suitable for long-time use for nourishing eyes, has the effects of preventing dry eyes and resisting fatigue and has excellent effects.
Owner:杭州光柱电子科技有限公司
Medicinal composition containing cefotiam hydrochloride
ActiveCN102716096BImprove stabilityImprove toleranceAntibacterial agentsPowder deliveryVitamin CMANNITOL/SORBITOL
The invention relates to a new medical preparation composition, in particular to an injection preparation containing cefotiam hydrochloride, and application of the injection preparation. The composition comprises cefotiam hydrochloride, anhydrous sodium carbonate, mannitol, vitamin C, and a sodium hydrogen citrate and trisodium citrate buffering solution.
Owner:哈药集团股份有限公司 +1
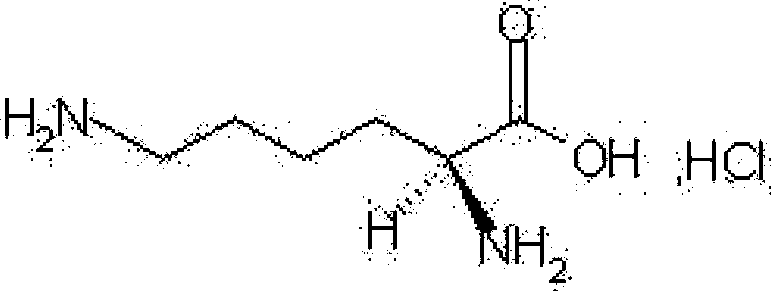
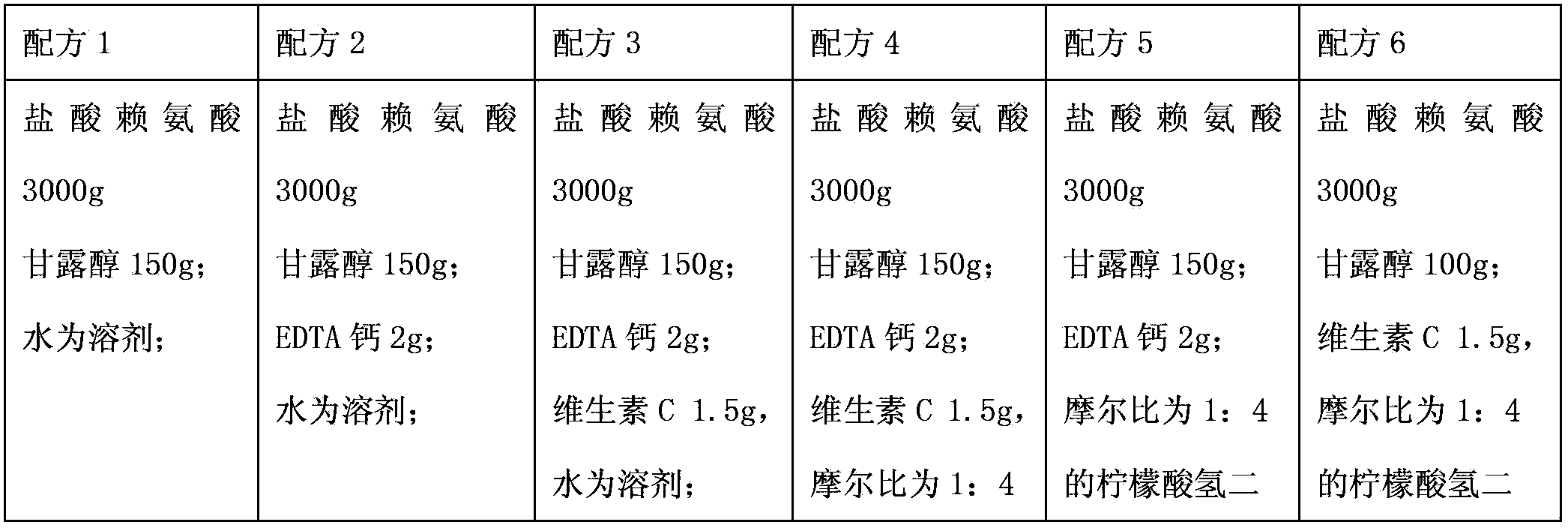
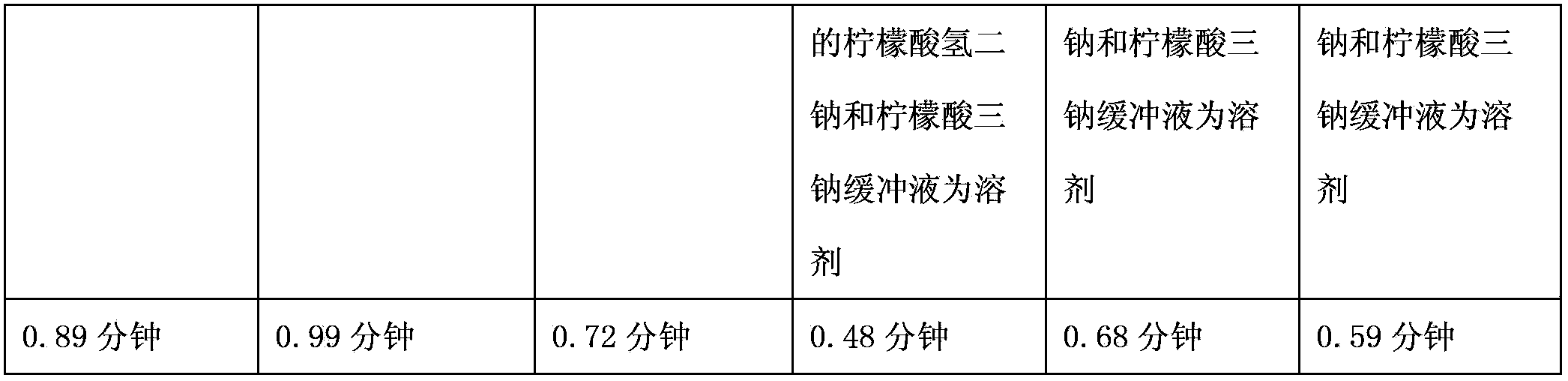
Medicinal composition containing lysine hydrochloride compound
ActiveCN102988304BImprove toleranceImprove solubilityPowder deliveryOrganic active ingredientsEthylene diamineVitamin C
The invention relates to a medicinal composition containing a lysine hydrochloride compound, and in particular relates to freeze-dried injection of lysine hydrochloride and a preparation method thereof. Each 1,000 injections are prepared from the following components: 3,000g of lysine hydrochloride, 100 to 200g of mannitol, 1 to 3g of ethylene diamine tetraacetic acid (EDTA) calcium, 1 to 2g of vitamin C, and 2,000ml of buffer solution of disodium hydrogen citrate and trisodium citrate in a mole ratio of 1:4.
Owner:姚云
Method for determining residual quantity of deltamethrin pesticide in tea leaves
InactiveCN105403642ASimple extraction methodGood effectComponent separationRelative standard deviationDeltamethrin
The invention relates to a method for determining the residual quantity of a deltamethrin pesticide in tea leaves. The method comprises the following steps: 1, preparing a sample: drying the tea leaves, grinding the dried tea leaves, and sieving the ground tea leaves; 2, carrying out deltamethrin crude extraction: placing the powdered tea leaves in a centrifuge tube, adding water to infiltrate the powdered tea leaves, adding petroleum ether and a mixed internal standard operating fluid, vibrating until uniformity, adding anhydrous magnesium sulfate, sodium chloride, sodium citrate, disodium hydrogen citrate and toluene, vibrating until uniformity, and centrifuging; 3, purifying a deltamethrin crude extraction product: taking a supernatant obtained in step 2 to the centrifuge tube, adding anhydrous magnesium sulfate and an N-propylethylene diamine bonded solid phase adsorbent, vibrating until uniformity, and centrifuging; 4, carrying out deltamethrin extraction: taking a supernatant obtained in step 3, carrying out Florisil silica column extraction by using a toluene-isooctane solution, concentrating, and filtering; and 5, carrying out chromatographic detection. The extraction method has the advantages of simplicity, good effect, and simple and accurate result integration treatment. The average recovery rate of the method is 93.21-103.75%, and the relative standard deviation is 3.28-6.20%, so the method accords with requirements, and is accurate and reliable.
Owner:周德志
Polymorphism of desloratadine disodium hydrogen citrate complex salt
ActiveCN103242292BLow dosage and non-toxicEasy to operateCarboxylic compound separation/purificationX-raySolvent
The invention belongs to the field of medicinal chemistry and specifically relates to a polymorphic and amorphous state of a desloratadine disodium hydrogen citrate complex salt and a preparation method thereof. The polymorphic and amorphous state is characterized in that an X-ray powder diffraction pattern of polymorphism has characteristic peaks in the vicinity of 2 theta angles of 11.45 degrees, 16.36 degrees, 17.74 degrees, 18.55 degrees, 19.13 degrees, 24.23 degrees, 27.54 degrees, 30.99 degrees, 32.82 degrees and 37.39 degrees. The preparation method of the polymorphic and amorphous state of the desloratadine disodium hydrogen citrate complex salt, disclosed by the invention, has the benefits of simplicity in operation, small using quantity of solvents, no toxicity and low production cost, and further has obvious advantages in the aspect of industrialization.
Owner:HEFEI AMVITE PHARM CO LTD +1
A flexible hydrosol adjuvant for ophthalmology
ActiveCN104814977BReduce turbidityReduce dosageSenses disorderAlkali/alkaline-earth metal chloride active ingredientsSodium acetateAdjuvant
The invention relates to medical preparations, in particular to a flexible hydrosol auxiliary agent for ophthalmology, which consists of: 0.2% to 0.5% of disodium hydrogen citrate, 1% to 1.5% of monosodium hydrogen citrate, and hydroxypropyl methylcellulose 5%~8%, sodium chondroitin sulfate 0.5%~1%, potassium chloride 0.5%~2%, magnesium chloride 0.3%~0.8%, sodium acetate 0.3%~1.5%, sodium chloride 1.3%~2.5%, no Bacteria double distilled water 85 ~ 90%. There can also be sodium hyaluronate 1% to 3%. The present invention is under the premise of ensuring the performance of the product, reducing the risk of nucleation and deposition, and slowing down the turbidity of the lens. Significantly reduced the consumption of disodium hydrogen citrate and magnesium chloride. It reduces the irritation to the patient's eyes, and avoids complications such as itching, tearing, redness and swelling in the patient's eyes. At the same time, the bionic level of the preparation is improved. The stability of the preparation is significantly improved, and it is suitable for long-term use, and has good effects as eye care, anti-dryness and anti-fatigue.
Owner:杭州光柱电子科技有限公司
Method for extracting and purifying hymexazol pesticide residues in tobacco and composition used
ActiveCN104181257BHigh extraction rateSimple stepsComponent separationPurification methodsPesticide residue
Owner:CHINA TOBACCO FUJIAN IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com