A flexible hydrosol adjuvant for ophthalmology
A flexible and hydrosol-based technology, applied in the active ingredients of alkali/alkaline earth metal chlorides, sensory diseases, drug combinations, etc., can solve the problem of reducing the interface energy between the compound and body fluid, increasing the surface electron density, and decreasing product stability, etc. problems, to reduce the risk of nucleation and deposition, slow down lens turbidity, and ensure the effect of performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
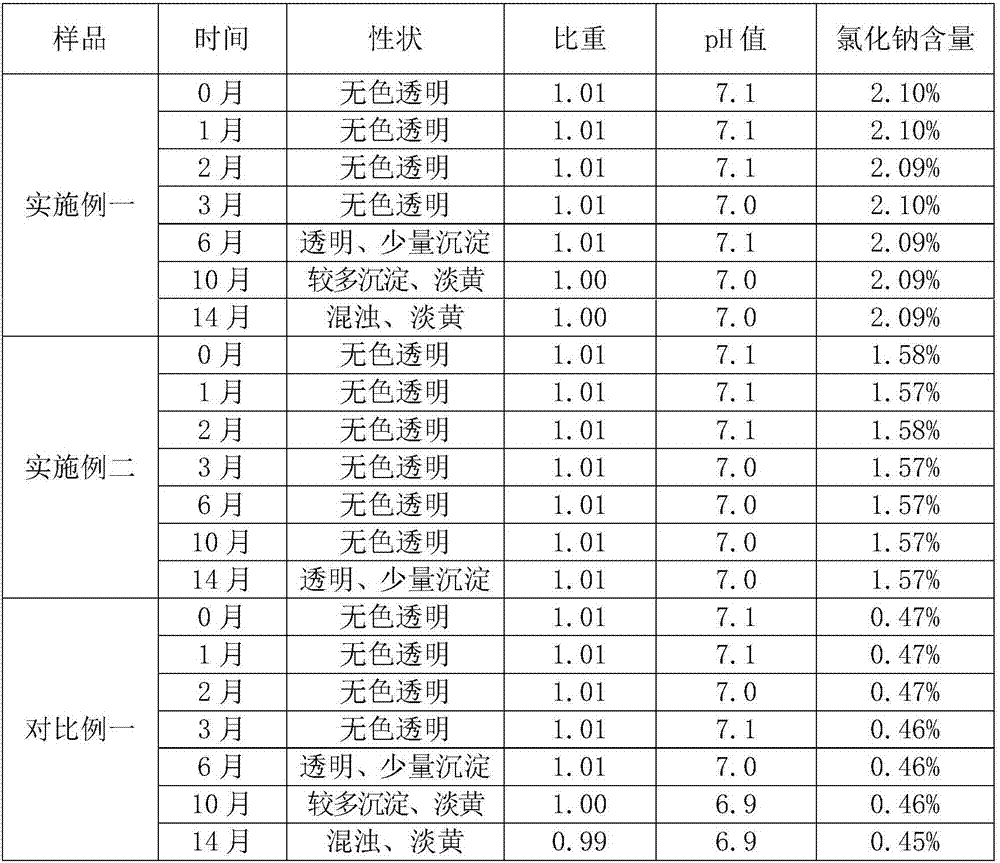
[0017] Take 40 g of sodium chloride and dissolve it in 1 liter of sterile double distilled water to make saline.
[0018] Under sterile conditions, mix 3 grams of disodium hydrogen citrate, 12 grams of monosodium hydrogen citrate, 70 grams of hydroxypropyl methylcellulose, 8 grams of sodium chondroitin sulfate, 10 grams of potassium chloride, 6 grams of magnesium chloride, Dissolve 10 grams of sodium acetate in 600 ml of saline, stir slowly at a constant speed for 5 minutes, then adjust the pH to 7.1 with hydrochloric acid or sodium hydroxide solution, and add sterile double distilled water to adjust to 1 liter.
[0019] Appearance of this product: completely transparent, specific gravity: 1.01.
Embodiment 2
[0021] Take 30 g of sodium chloride and dissolve it in 1 liter of sterile double distilled water to make saline.
[0022] Under sterile conditions, mix 3 grams of disodium hydrogen citrate, 12 grams of monosodium hydrogen citrate, 50 grams of hydroxypropyl methylcellulose, 15 grams of sodium hyaluronate, 8 grams of sodium chondroitin sulfate, 15 grams of chlorine Potassium chloride, 6 grams of magnesium chloride, and 15 grams of sodium acetate were dissolved in 600 milliliters of brine, stirred slowly at a constant speed for 5 minutes, and then the pH was adjusted to 7.1 with hydrochloric acid or sodium hydroxide solution, and then adjusted to 1 liter with sterile double-distilled water. have to.
[0023] Appearance of this product: completely transparent, specific gravity 1.02.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com