Medicinal composition containing cefotiam hydrochloride
A cefotiam and composition technology, applied in the field of new pharmaceutical preparation compositions, can solve the problems of unsatisfactory dissolution rate, increase dissolution rate, stability problems, etc., achieve rapid dissolution, ensure stability, and tolerate good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
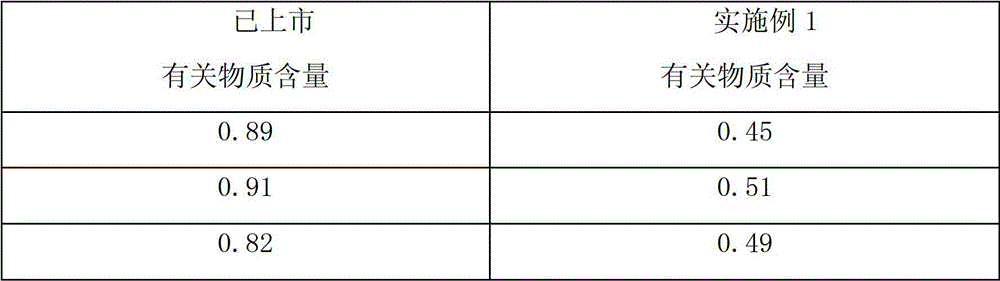
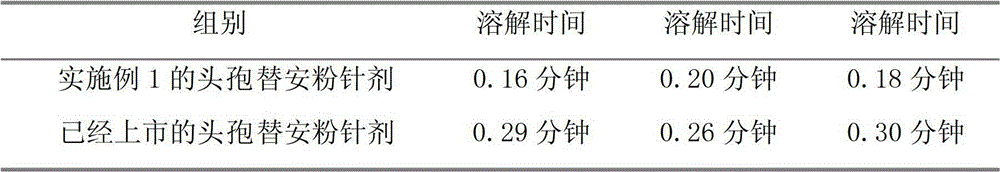
Examples
Embodiment 1
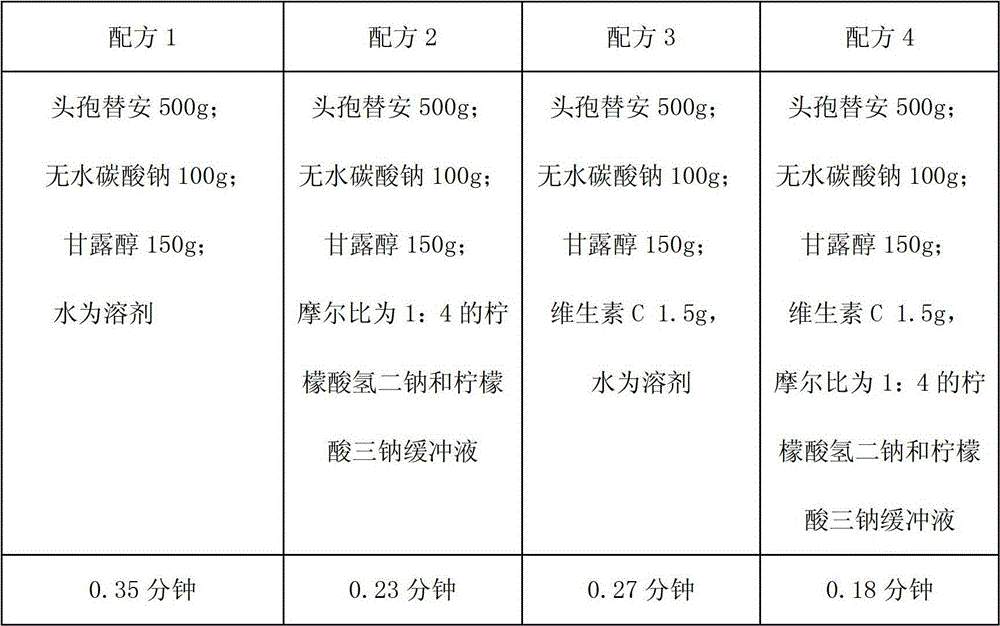
[0051] Cefotiam 500g;
[0052] Anhydrous sodium carbonate 100g;
[0053] Mannitol 150g;
[0054] Vitamin C1.5g,
[0055] 2000ml of disodium hydrogen citrate and trisodium citrate buffer solution with a molar ratio of 1:4,
[0056] Add water for injection to 4000ml
[0057] 1000 bottles
[0058] Preparation: First mix 2000ml of disodium hydrogen citrate and trisodium citrate buffer solution, then take the above-mentioned amount of cefotiam, mannitol, vitamin C and dissolve in citric acid buffer solution, then dilute to 4000ml with water for injection, pass through the aperture Filtrate through a 0.22um filter membrane, after the clarity is qualified, fill, half-tighten, freeze-dry in a box, pack, and get ready. Wherein, the preparation method of disodium hydrogen citrate and trisodium citrate buffer solution is as follows: weigh disodium hydrogen citrate 13g, trisodium citrate 60g and dissolve in 2000ml water for injection to obtain a buffer solution with a...
Embodiment 2
[0060] Cefotiam 1000g;
[0061] Anhydrous sodium carbonate 200g;
[0062] Mannitol 200g;
[0063] Vitamin C 2g,
[0064] Disodium hydrogen citrate and trisodium citrate buffer solution with a molar ratio of 1:4 2000ml
[0065] Add water for injection to 4L
[0066] 1000 bottles
[0067]Preparation: first mix 1000ml of disodium hydrogen citrate and trisodium citrate buffer solution, then take the above amount of cefotiam, mannitol, EDTA calcium, vitamin C and citric acid buffer solution to dissolve, and then dilute to 4000ml with water for injection , filtered through a filter membrane with a pore size of 0.22um, and after the clarity is qualified, it is filled, half-pressed, put into a box for freeze-drying, and packaged to obtain the product. Wherein, the preparation method of disodium hydrogen citrate and trisodium citrate buffer solution is as follows: weigh disodium hydrogen citrate 13g, trisodium citrate 60g and dissolve in 2000ml water for injectio...
Embodiment 3
[0069] Cefotiam 500g;
[0070] Anhydrous sodium carbonate 120;
[0071] Mannitol 100g;
[0072] Vitamin C1g,
[0073] Disodium hydrogen citrate and trisodium citrate buffer solution with a molar ratio of 1:4 2000ml
[0074] Add water for injection to 4L
[0075] 1000 bottles
[0076] Preparation: First mix 2000ml of disodium hydrogen citrate and trisodium citrate buffer solution, then take the above-mentioned amount of cefotiam, mannitol, vitamin C and dissolve in citric acid buffer solution, then dilute to 4000ml with water for injection, pass through the aperture Filtrate through a 0.22um filter membrane, after the clarity is qualified, fill, half-tighten, freeze-dry in a box, pack, and get ready. Wherein, the preparation method of disodium hydrogen citrate and trisodium citrate buffer solution is as follows: weigh disodium hydrogen citrate 13g, trisodium citrate 60g and dissolve in 2000ml water for injection to obtain a buffer solution with a pH value of 7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com