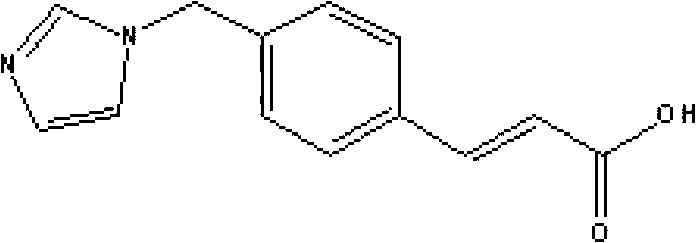
Medicinal composition containing sodium ozagrel compound
A technology of sodium ozagrel and its composition, which is applied in the field of freeze-dried powder injection of sodium ozagrel and its preparation, can solve the problems of inconvenient use and unsatisfactory dissolution speed, and achieve convenient use, rapid dissolution and stability Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
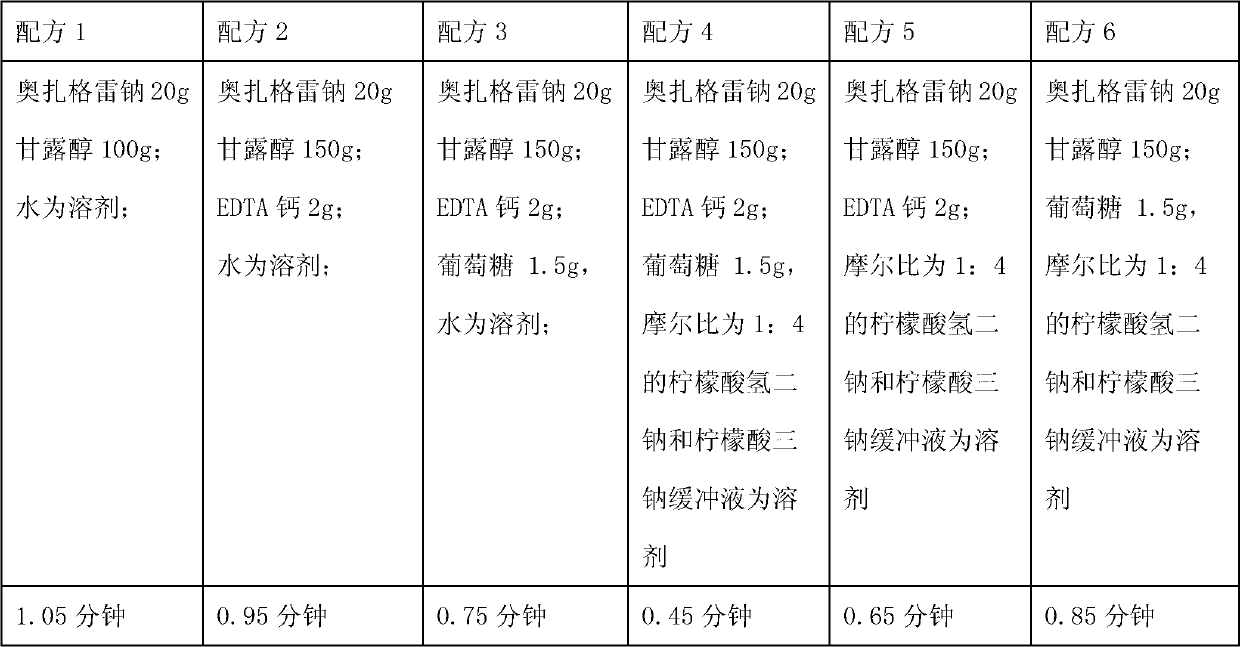
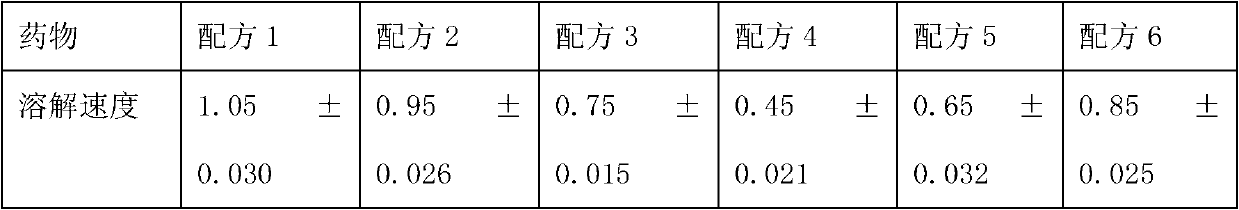
[0061] Ozagrel sodium 20g;
[0062] Mannitol 150g;
[0063] Calcium EDTA 2g;
[0064] Glucose 1.5g,
[0065] 2000ml of disodium hydrogen citrate and trisodium citrate buffer solution with a molar ratio of 1:4;
[0066] Preparation
[0067] (1) Weigh the raw and auxiliary materials according to the prescription quantity
[0068] (2) Add mannitol, EDTA calcium, and glucose to 1000ml of disodium hydrogen citrate and trisodium citrate buffer solution with a molar ratio of 1:4, and stir to dissolve.
[0069] (3) Add the prescribed amount of sodium ozagrel into the solution and stir to dissolve completely. Add disodium hydrogen citrate and trisodium citrate buffer with a molar ratio of 1:4 to the full amount, and stir to mix evenly.
[0070] (4) Measure the pH of the solution to be 7.0.
[0071] (5) Fine filter with a 0.2 μm microporous membrane to check the clarity of the solution.
[0072] (6) According to the measurement results, fill the liquid medicine into a vial with ...
Embodiment 2
[0076] Ozagrel sodium 20g;
[0077] Mannitol 100g;
[0078] Calcium EDTA 1g;
[0079] Glucose 1g,
[0080] 2000ml of disodium hydrogen citrate and trisodium citrate buffer solution with a molar ratio of 1:4;
[0081] Preparation
[0082] (1) Weigh the raw and auxiliary materials according to the prescription quantity
[0083] (2) Add mannitol, EDTA calcium, and glucose to 1000ml of disodium hydrogen citrate and trisodium citrate buffer solution with a molar ratio of 1:4, and stir to dissolve.
[0084] (3) Add the prescribed amount of sodium ozagrel into the solution and stir to dissolve completely. Add disodium hydrogen citrate and trisodium citrate buffer with a molar ratio of 1:4 to the full amount, and stir to mix evenly.
[0085] (4) Measure the pH value of the solution, which is 7.0.
[0086] (5) Fine filter with a 0.2 μm microporous membrane to check the clarity of the solution.
[0087] (6) According to the measurement results, fill the liquid medicine into a vial...
Embodiment 3
[0091] Ozagrel sodium 20g;
[0092] Mannitol 200g;
[0093] Calcium EDTA 3g;
[0094] Glucose 2g,
[0095] 2000ml of disodium hydrogen citrate and trisodium citrate buffer solution with a molar ratio of 1:4;
[0096] Preparation
[0097] (1) Weigh the raw and auxiliary materials according to the prescription quantity
[0098] (2) Add mannitol, EDTA calcium, and glucose to 1000ml of disodium hydrogen citrate and trisodium citrate buffer solution with a molar ratio of 1:4, and stir to dissolve.
[0099] (3) Add the prescribed amount of sodium ozagrel into the solution and stir to dissolve completely. Add disodium hydrogen citrate and trisodium citrate buffer with a molar ratio of 1:4 to the full amount, and stir to mix evenly.
[0100] (4) Measure the pH value of the solution, which is 7.0.
[0101] (5) Fine filter with a 0.2 μm microporous membrane to check the clarity of the solution.
[0102] (6) According to the measurement results, fill the liquid medicine into a vi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com