Sodium ozagrel freeze-dried powder for injection and preparation method of sodium ozagrel freeze-dried powder
A technology of ozagrel sodium and freeze-dried powder, which is applied in the field of ozagrel sodium freeze-dried powder for injection and its preparation, and can solve the problem of unstable quality of ozagrel sodium freeze-dried powder for injection and non-standardized step control process , Increase the risk of clinical use and other issues, to achieve the effect of improving the safety of clinical use, suitable for promotion, and high safety in clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The invention provides a freeze-dried powder of ozagrel sodium for injection, which comprises 70 g of ozagrel, 150 g of mannitol and 10 g of sodium hydroxide in parts by weight.
[0043] The preparation method of ozagrel sodium freeze-dried powder for injection comprises the following steps:
[0044] S1 formula preparation;
[0045] S2 liquid preparation:
[0046] S2-1: Disperse ozagrel in water for injection, slowly add sodium hydroxide while stirring, and stir until ozagrel is completely dissolved to obtain a clear liquid;
[0047] S2-2: Add mannitol and stir until completely dissolved;
[0048] S2-3: Adjust the pH value to 8.2 with sodium hydroxide solution, which effectively inhibits the hydrolysis of the formula and improves the stability of the drug;
[0049] S2-4: Add medicinal activated carbon, stir for 30 minutes to make it evenly dispersed, successively pass through a 0.45 μm microporous membrane for filtration decarbonization, and pass through a 0.22 μm mi...
Embodiment 2 Embodiment 9
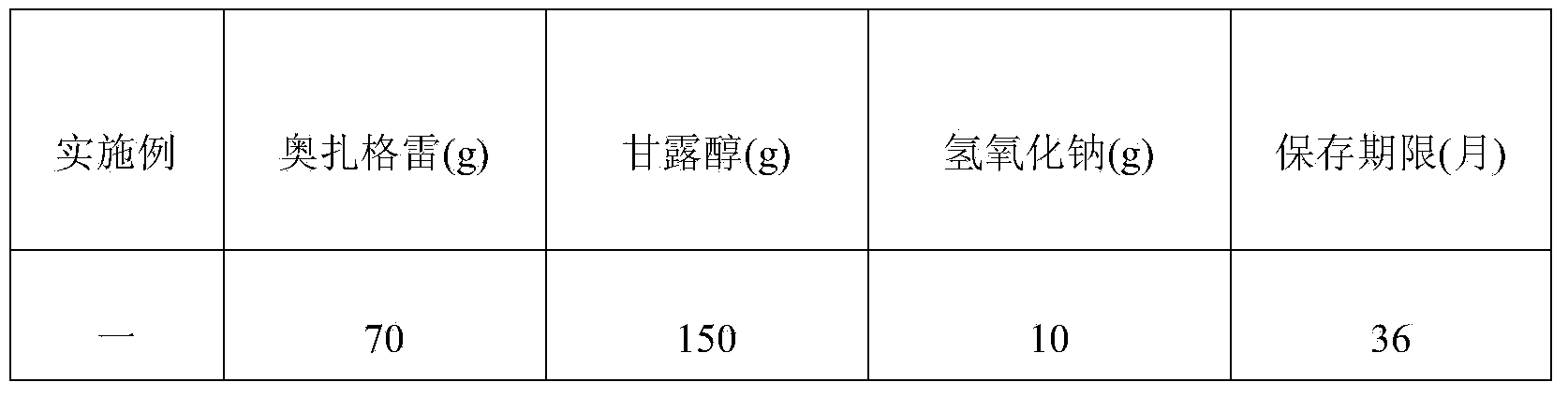
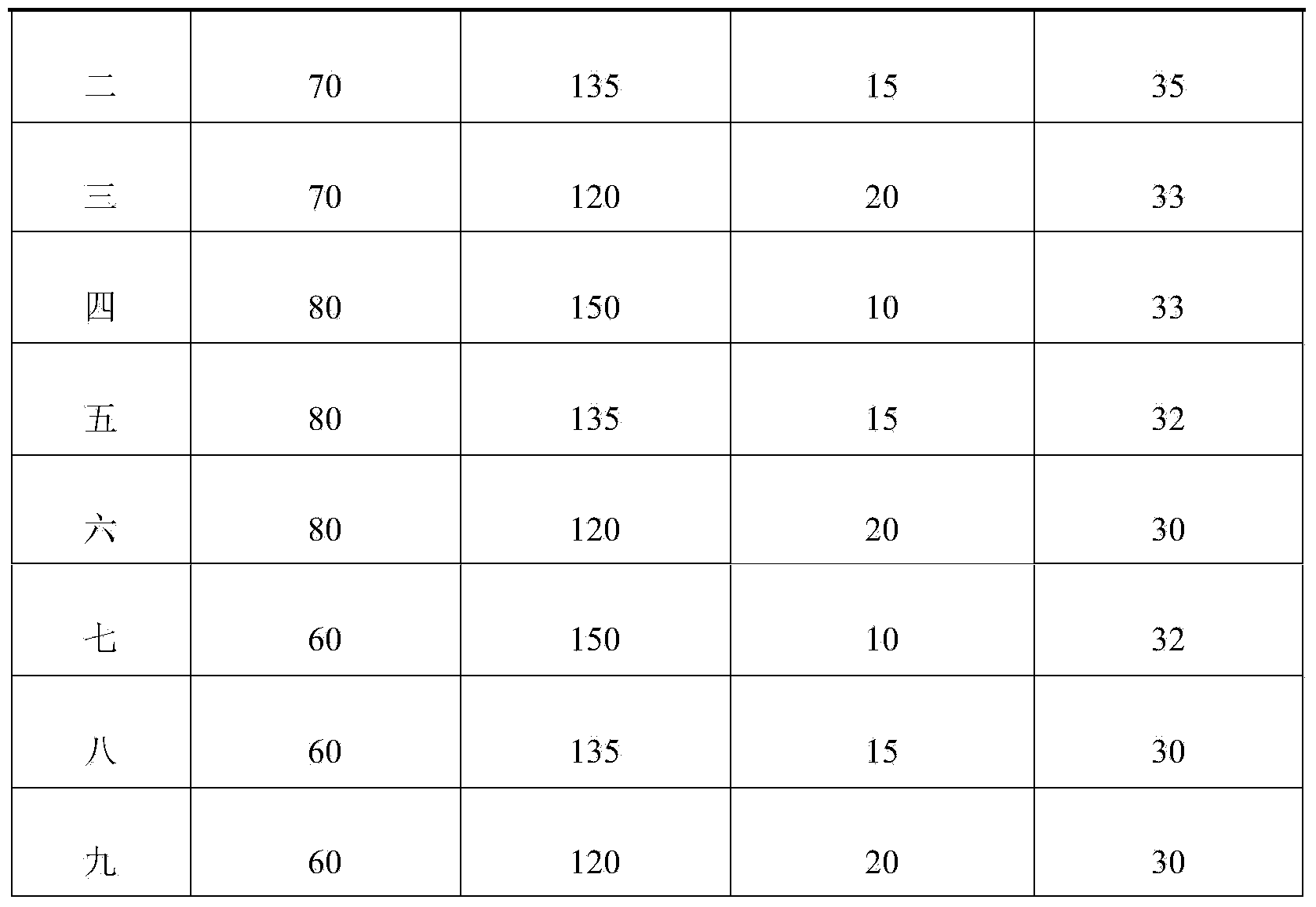
[0064] In Example 1 to Example 9, the composition formula of ozagrel sodium freeze-dried powder for injection is shown in Table 1, and its preparation method is the same as that of Example 1. Continue to carry out the stability study, as can be seen from the data in Table 1, the obtained ozagrel sodium freeze-dried powder for injection has high quality, good stability, low water content in the finished product, and a shelf life of more than 30 months. Especially in Example 1, when the composition formula of ozagrel sodium lyophilized powder for injection is ozagrel 70g, mannitol 150g and sodium hydroxide 10g, the stability of the obtained ozagrel sodium lyophilized powder for injection is the best , the retention period can reach 36 months.
[0065] Table 1 Ozagrel Sodium Freeze-Dried Powder for Injection with Different Formulations
[0066]
[0067]
Embodiment 10 Embodiment 12
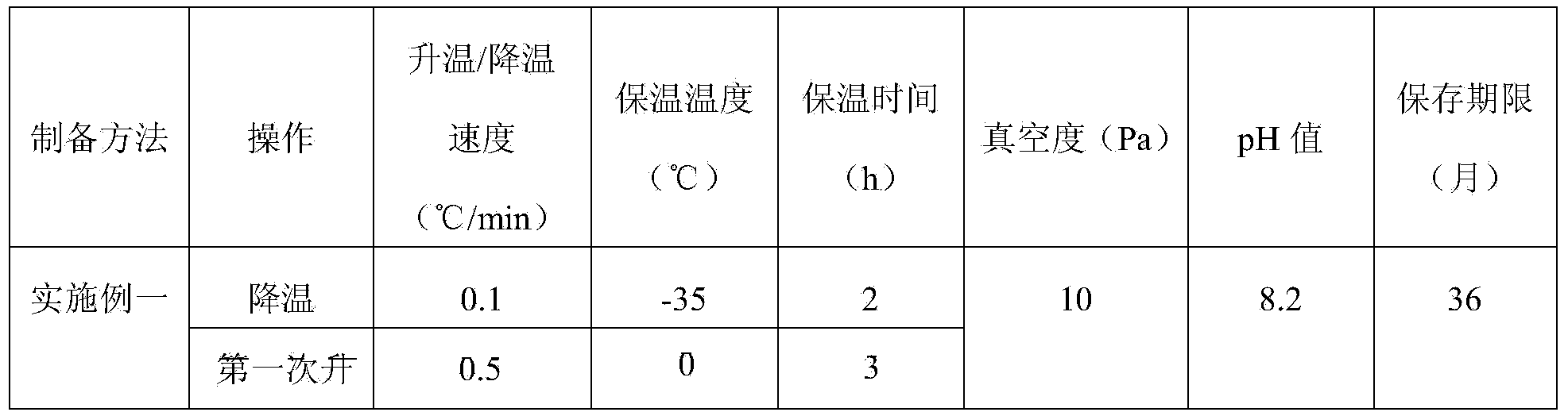
[0069] The composition and formula of the ozagrel sodium freeze-dried powder for injection in the tenth to the twelveth embodiment are all the same as in the first embodiment, and the preparation method is to reduce the pH value in the step S2-3, and the cooling in the step S4-1 to S4-4. , the first temperature rise, the temperature rise rate of the second temperature rise, the holding temperature, the holding time and the vacuum degree are replaced with the values shown in Table 2, and the stability research of the obtained finished product is continued at room temperature, and the results are shown in Table 2 As shown in, compared with the prior art, the method of the present invention adopts the vacuum low-temperature freeze-drying process to prepare the medicine in an airtight container, so as to ensure that the medicine is not easy to oxidize and deteriorate, and overcome the problem of medicine decomposition caused by high temperature in the production process, and the o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com