Preparation method of Ozagrel sodium
A technology of sodium ozagrel and sodium hydroxide, which is applied in the field of medicine, can solve the problems that acetone is easy to produce poison, the yield and purity of ethyl ozagrel are affected, and the synthesis reaction time of ethyl imidazole methyl cinnamate is long, and the like, To achieve the effect of improving yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
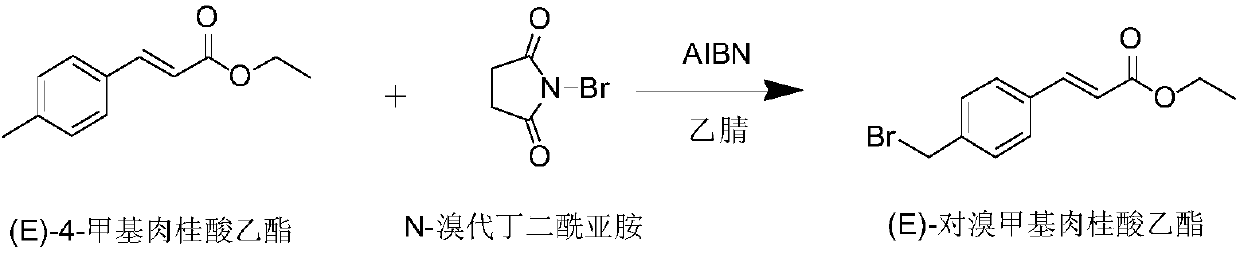
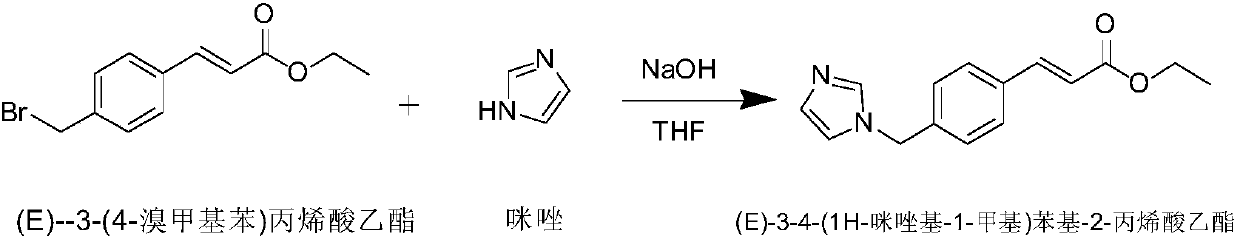
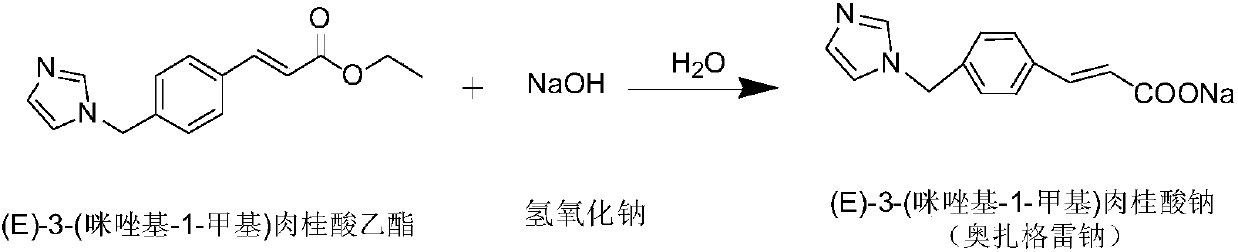
[0034] A kind of preparation method of ozagrel sodium, concrete steps are as follows:
[0035] (1) Put 20g of ethyl p-methylcinnamate in a three-necked flask, add 0.05g of azoisobutyronitrile, 50ml of acetonitrile, and heat to 65°C; then add 20.6g of N-bromosuccinimide , warming up to 80° C., reflux reaction for 2 hours, sampling, detection (HPLC) purity of ethyl p-bromomethyl cinnamate: 81.01%, stop the reaction to obtain reaction solution I.
[0036] After the reaction, the solvent acetonitrile (60° C.) of the reaction solution I was vacuum distilled to obtain an oily substance (containing solid granular substances, mainly succinimide); 40 ml of ethyl acetate was added to the oily substance, and stirred for 0.5 h Afterwards, filter, filter out the insoluble matter (succinimide), wash the solid with a small amount of ethyl acetate, add 40ml of water to the ethyl acetate, stir for 15min, let stand to separate the layers, separate the water layer and the ethyl acetate layer ; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com