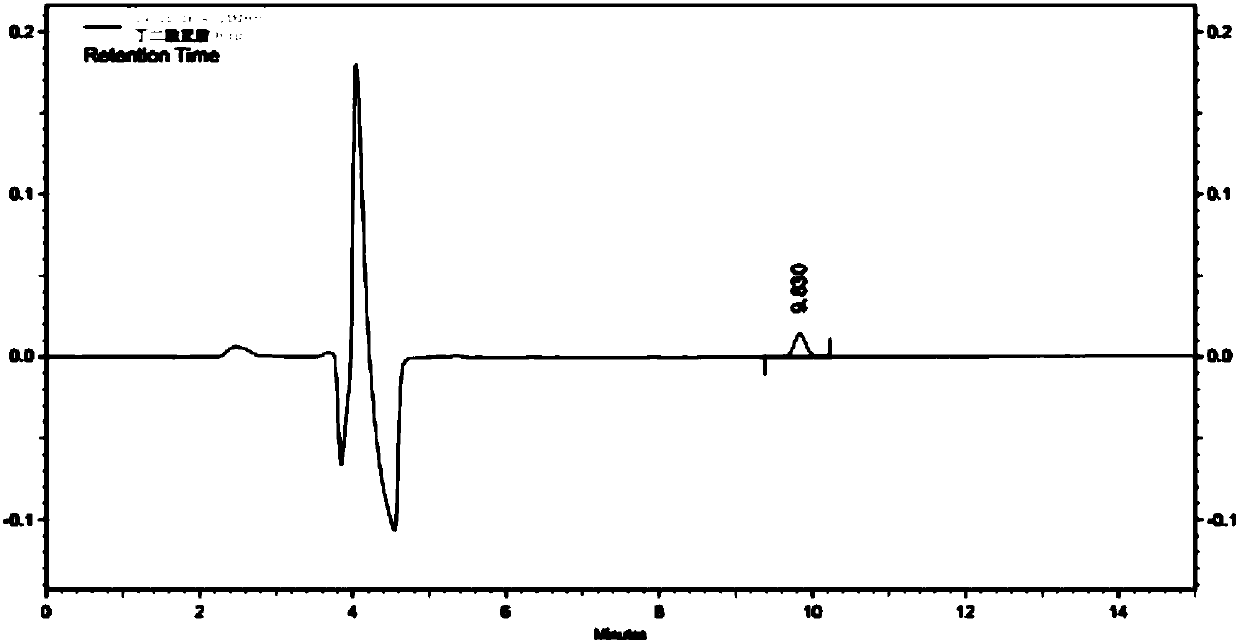
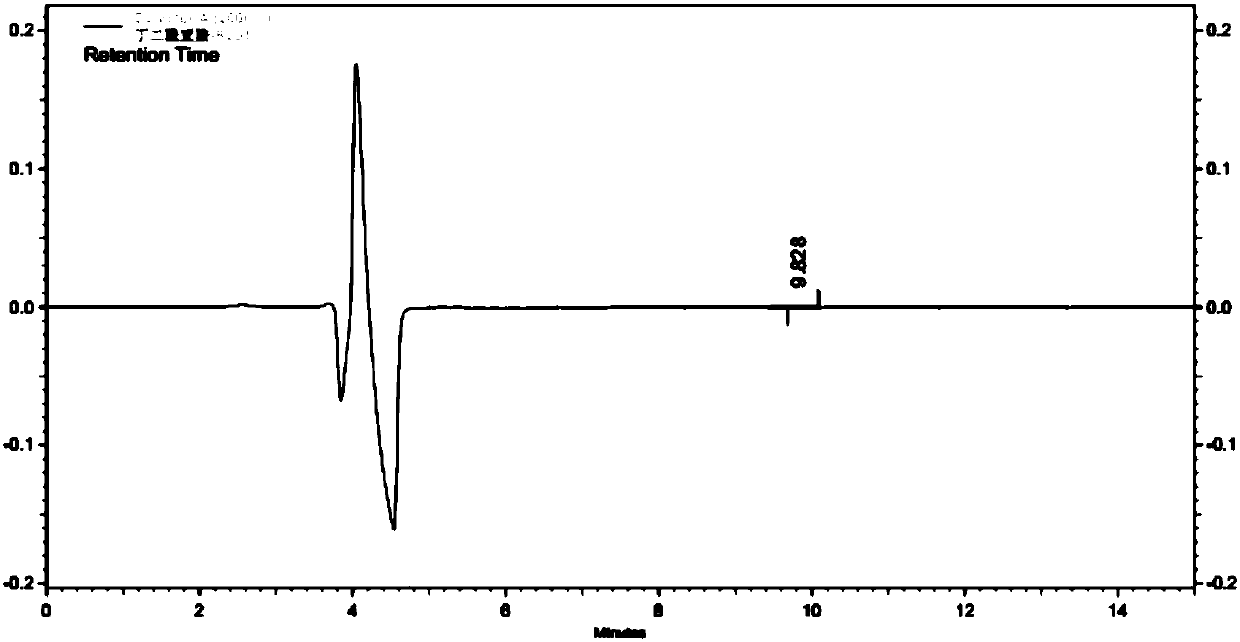
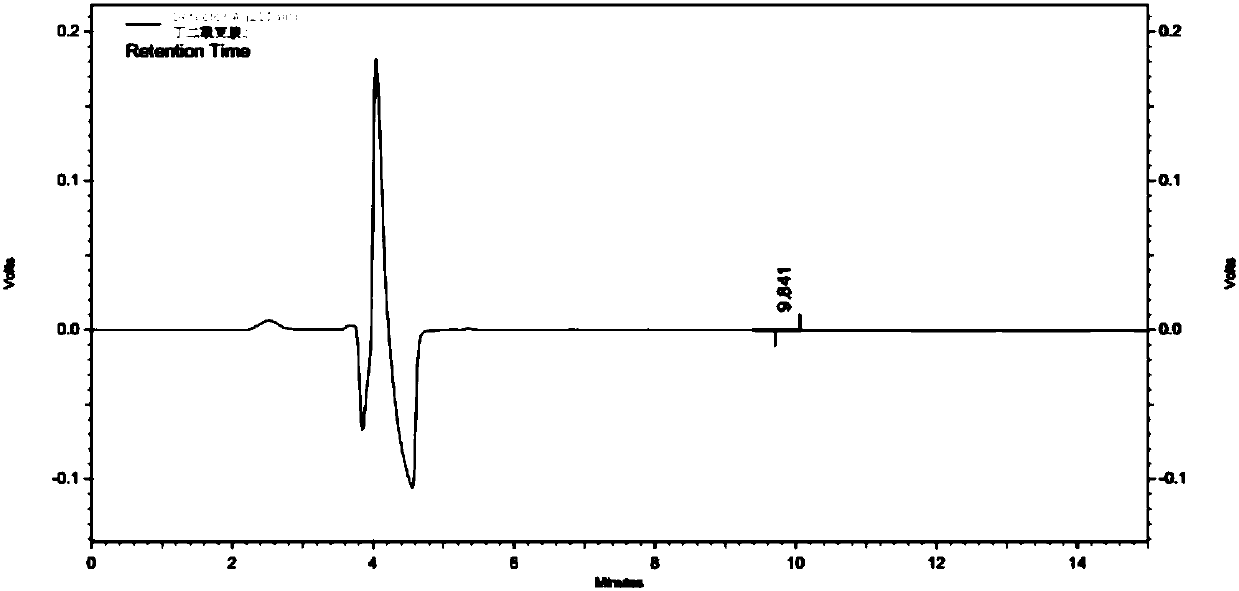
Method for detecting content of bromo-succinimide and succinimide in sodium ozagrel raw material medicine
The technology of bromosuccinimide and succinimide is applied in the field of medicine, and can solve the problems of instability, residue of N-bromosuccinimide, affecting the quality of sodium ozagrel injection, and the like, Achieving the effect of ensuring quality and good quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A method for detecting content of bromosuccinimide and succinimide in ozagrel sodium crude drug, the specific steps are as follows:
[0029] (1) Get the sodium ozagrel sample to be measured, add 0.05mol / L hydrochloric acid aqueous solution to dissolve and dilute to make a solution containing about 1mg sodium ozagrel sample in every 1ml, as need testing solution;
[0030] (2) get succinimide standard substance, dissolve and dilute with 0.05mol / L hydrochloric acid aqueous solution and make the solution that contains about 1 μ g succinimide standard substance in every 1ml, as reference substance solution;
[0031] (3) Utilize high-performance liquid chromatography to detect succinimide in the test solution; wherein, adopt CAPCELLPAK C18 AQ (250mm × 4.6mm, 5 μm) chromatographic column; mobile phase A is 0.02mol / L dihydrogen phosphate Potassium (use 10% potassium hydroxide to adjust the pH value to 6.5), the mobile phase B is methanol, perform linear gradient elution in the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com