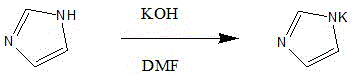Preparation process of ozagrel intermediate methyl (E)-4-(imidazolylmethyl) cinnamate
A technology of imidazolyl methyl and methyl cinnamate, which is applied in the field of preparation of ozagrel intermediate-methyl 4-cinnamate, can solve the impact of ozagrel cycle and cost, complicated process of removing impurities, and imidazolium salts. The preparation process is cumbersome and other problems, to achieve the effect of shortening the production cycle, short production cycle and easy purchase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. Preparation of Potassium Imidazole:
[0039] Put 63kg of DMF into a 500L reactor, put in 12.7kg of potassium hydroxide and 15.5kg of imidazole, stir at 10-20°C for 1 hour to obtain a mixed solution; react at 15°C for 3 hours; drop to below 0°C to obtain a spare material ;
[0040] 2. Preparation of methyl (E)-4-(imidazolylmethyl)cinnamate:
[0041] Dissolve 50 kg of methyl p-bromomethyl cinnamate in 55 kg of DMF, control the temperature of the methyl p-bromomethyl cinnamate solution below 15°C and add it dropwise, and continue stirring for 4 hours after dropping; add 300 kg of deionized Stir and crystallize with water, and dry at 60-70°C for 23 hours after rejection filtration to obtain 37.3kg of (E)-4-(imidazolylmethyl)cinnamic acid methyl ester. The yield is 78.5%, the purity is 99.24%, and the appearance is off-white flaky solid.
Embodiment 2
[0043] 1, the preparation of potassium imidazole:
[0044] Put 125 kg of DMF into a 1000L reactor, put in 25.4 kg of potassium hydroxide and 31 kg of imidazole, stir at 14°C for 1 hour; add 60 kg of sodium carbonate, and react at 14°C for 5 hours; ℃ below to obtain spare materials.
[0045] 2. Preparation of methyl (E)-4-(imidazolylmethyl)cinnamate:
[0046] Dissolve 100 kg of methyl p-bromomethyl cinnamate in 110 kg of DMF, control the temperature of the methyl p-bromomethyl cinnamate solution below 15°C and drop in, and continue stirring for 5 hours after dropping; add 600 kg of deionized Stir and crystallize with water, shake off and dry at 60-70°C for 24 hours to obtain 75.5kg of (E)-4-(imidazolylmethyl)cinnamic acid methyl ester. The yield is 79.5%, the purity is 99.2%, and the appearance is off-white flaky solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com