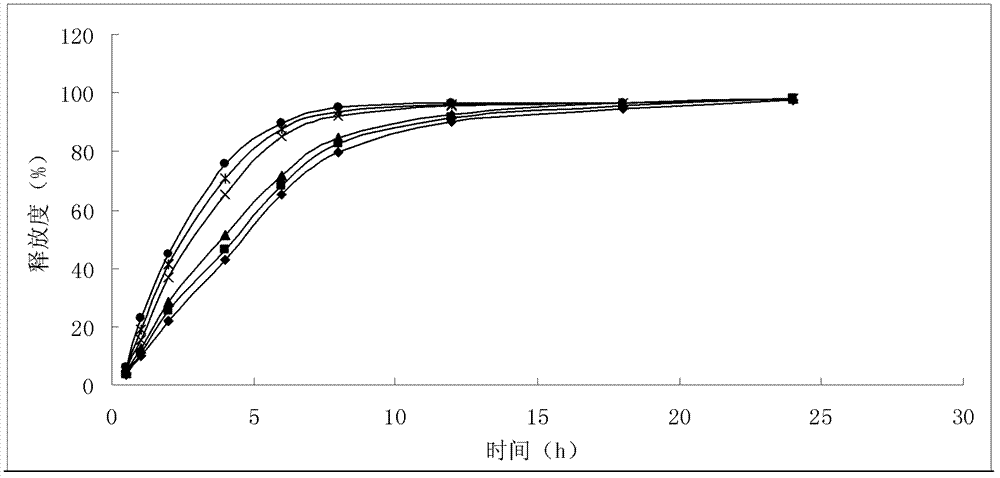
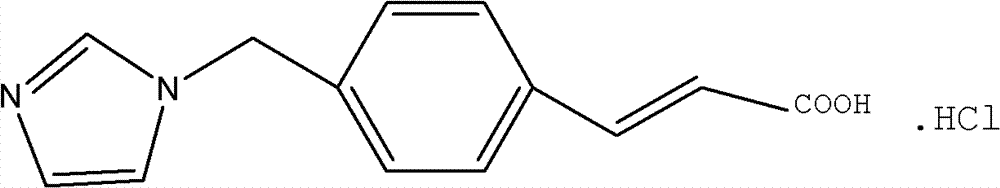
Liposome solid preparation of ozagrel
A technology of ozagrel hydrochloride and liposomes, which is applied in the field of medicine, can solve the problems of low bioavailability, long dissolution time, and affecting the therapeutic effect, and achieve high bioavailability, excellent dissolution, and good sustained-release effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1 Ozagrel Hydrochloride Liposome Tablets
[0074] The raw and auxiliary materials used are as follows:
[0075]
[0076] Adopt the following production process to prepare ozagrel hydrochloride liposome tablet:
[0077] (1) Accurately weigh 100g of ozagrel hydrochloride, 160g of hydrogenated egg yolk lecithin, 80g of dioleoylphosphatidylethanolamine, 120g of cholesterol succinate, and 40g of Span 20, and dissolve them in 3000ml of methanol and tert- In the butanol mixed solvent, stir to make it dissolve;
[0078] (2) Place the above solution in an eggplant-shaped bottle, remove methanol and tert-butanol under reduced pressure in a 45°C water bath, and form a uniform transparent film on the wall of the bottle;
[0079] (3) Add 10000ml of phosphate buffer solution with a pH value of 6.8 to the eggplant-shaped bottle, and continue to rotate in a water bath at 45°C under normal pressure to swell and hydrate the film;
[0080] (4) Filter the above solution w...
Embodiment 2
[0084] Example 2 Ozagrel Hydrochloride Liposome Tablets
[0085] The raw and auxiliary materials used are as follows:
[0086]
[0087] Adopt the following production process to prepare ozagrel hydrochloride liposome tablet:
[0088] (1) Accurately weigh 200g of ozagrel hydrochloride, 280g of hydrogenated egg yolk lecithin, 140g of dioleoylphosphatidylethanolamine, 210g of cholesterol succinate, 40g of Span 20, and dissolve them in 5000ml of methanol and t- In the butanol mixed solvent, stir to make it dissolve;
[0089] (2) Place the above solution in an eggplant-shaped bottle, remove methanol and tert-butanol under reduced pressure in a 45°C water bath, and form a uniform transparent film on the wall of the bottle;
[0090] (3) Add 15000ml of phosphate buffer solution with a pH value of 6.8 to the eggplant-shaped bottle, and continue to rotate in a water bath at 45°C under normal pressure to swell and hydrate the film;
[0091] (4) Filter the above solution with a ...
Embodiment 3
[0095] Example 3 Ozagrel Hydrochloride Liposome Tablets
[0096] The raw materials used are as follows:
[0097]
[0098] Adopt following production process to prepare ozagrel hydrochloride liposome tablet:
[0099] (1) Accurately weigh 200g of ozagrel hydrochloride, 240g of hydrogenated egg yolk lecithin, 120g of dioleoylphosphatidylethanolamine, 180g of cholesterol succinate, 80g of Span 20 and dissolve them in 4000ml of methanol and tert-butyl at a volume ratio of 2:1. In the mixed solvent of alcohol, stir to make it dissolve;
[0100] (2) Place the above solution in an eggplant-shaped bottle, remove methanol and tert-butanol under reduced pressure in a 45°C water bath, and form a uniform transparent film on the wall of the bottle;
[0101] (3) Add 12000ml of phosphate buffer solution with a pH value of 6.8 to the eggplant-shaped bottle, and continue to rotate in a water bath at 45°C under normal pressure to make the film swell and hydrate;
[0102] (4) Filter the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com