Olopatadine hydrochloride eye drop and preparation method thereof
A technology of olopatadine hydrochloride and olopatadine, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as easy recurrence, achieve improved stability, high stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
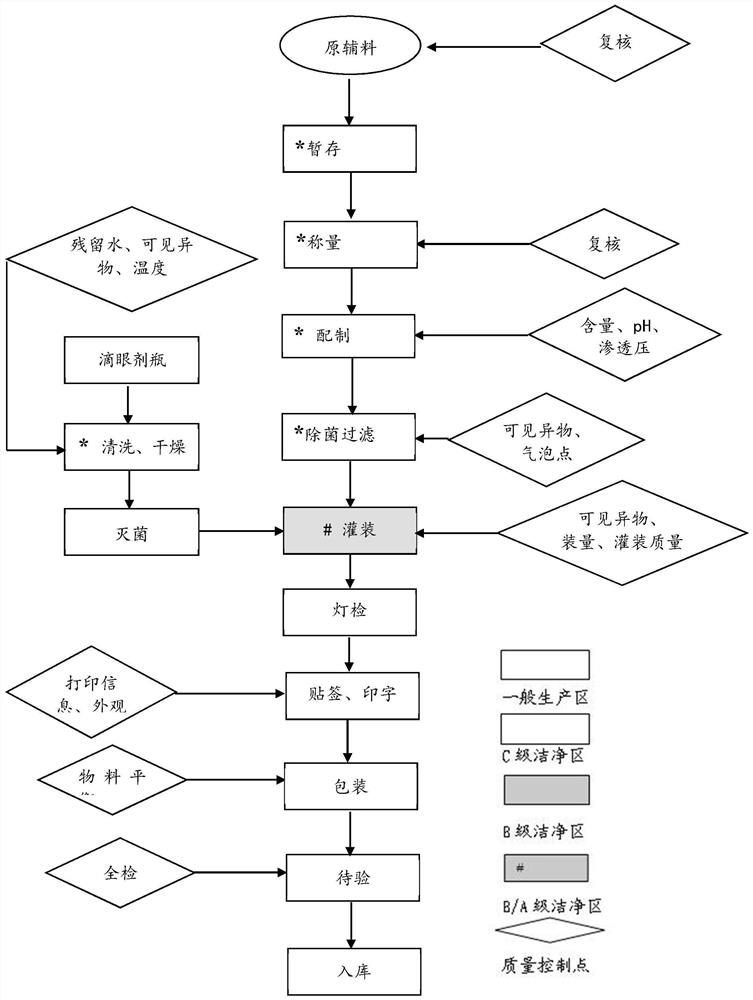
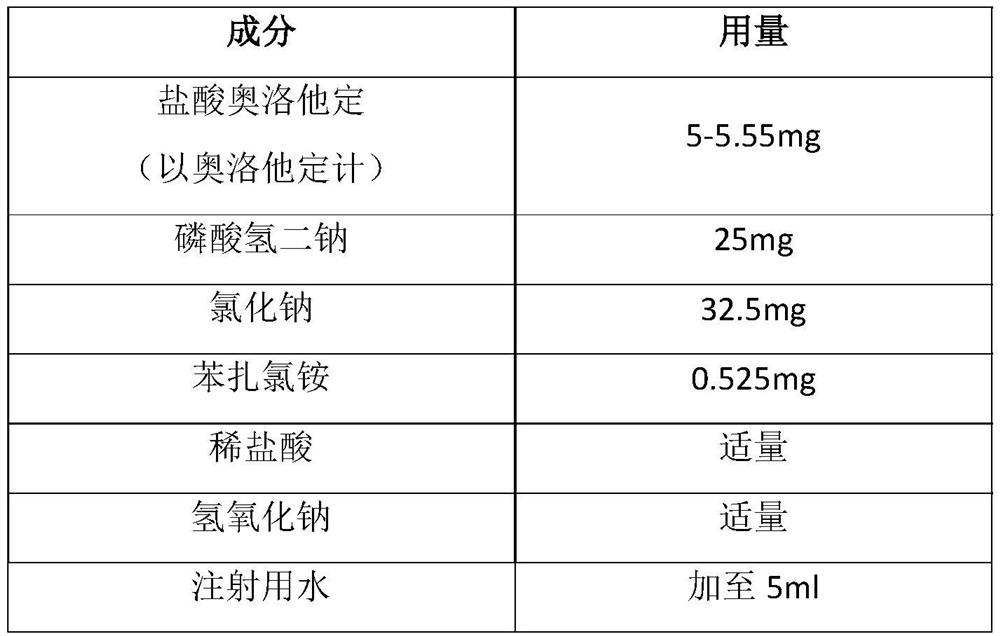
[0064] Embodiment 1: prepare olopatadine hydrochloride eye drops (as figure 1 shown)
[0065] ①Bottle washing, stopper washing and sterilization
[0066] To receive the bottle used for olopatadine hydrochloride eye drops of 5ml size, start the XH2S60 vertical bottle washing machine according to the "Standard Operating Procedure for Eye Drop Bottle Washing Procedure", and wash the bottle with water for injection filtered through a 0.22μm filter element, for injection The water pressure is ≥0.2MPa, the circulating water pressure is ≥0.2MPa, the compressed air pressure is ≥0.2MPa, and the circulating water temperature is 30-50°C. Check it every 2 hours. Dry after cleaning. The drying temperature is set at 60°C, the temperature is controlled at 55-68°C, and the drying section maintains positive pressure. The dried bottles are packed into double-layer polyethylene plastic bags in 2500 pieces / bag, and the deviation of the number of bottles in each bag is controlled within 10%. Sq...
Embodiment 2
[0088] The detection method of the olopatadine hydrochloride eye drops described in embodiment 1, the method comprises:
[0089] 1. Detection of related substances
[0090] 1) Polar impurities
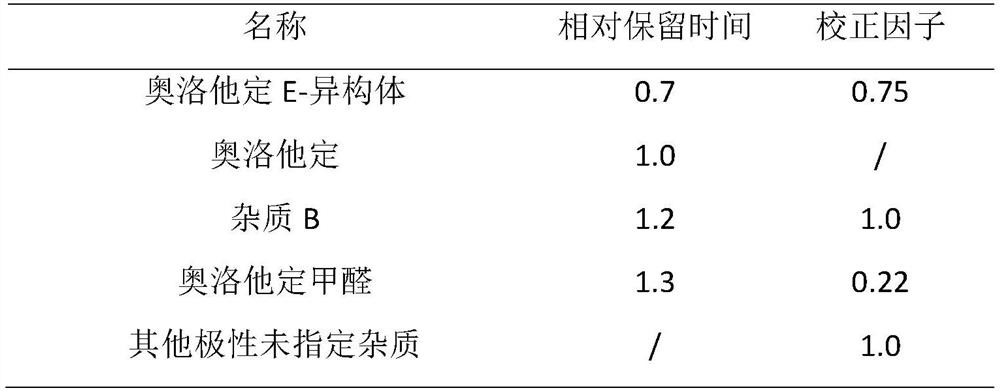
[0091] f) Accurately weigh an appropriate amount of olopatadine hydrochloride reference substance, dissolve and dilute it with mobile phase to make a solution containing about 0.2 mg of olopatadine hydrochloride in every 1 ml, as the stock solution of olopatadine hydrochloride reference substance, measure 10 ml, Add 1ml of 30% hydrogen peroxide solution, mix well, add 2 drops of 50% sodium hydroxide solution, and let it stand for more than 30 minutes to obtain the impurity B control solution; take another olopatadine E-isomer and olopatadine An appropriate amount of formaldehyde impurity reference substance was dissolved with mobile phase and diluted to a solution containing 20 μg per 1 ml, which was used as impurity reference substance stock solution; measure 3ml of impurity B contro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com