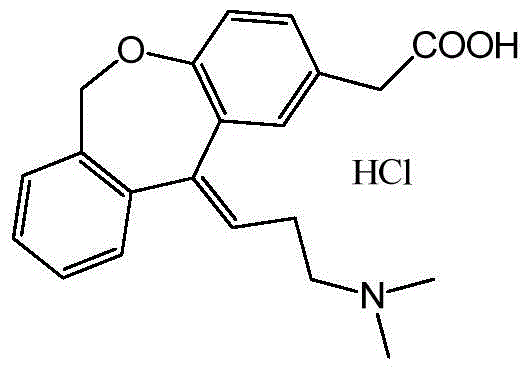
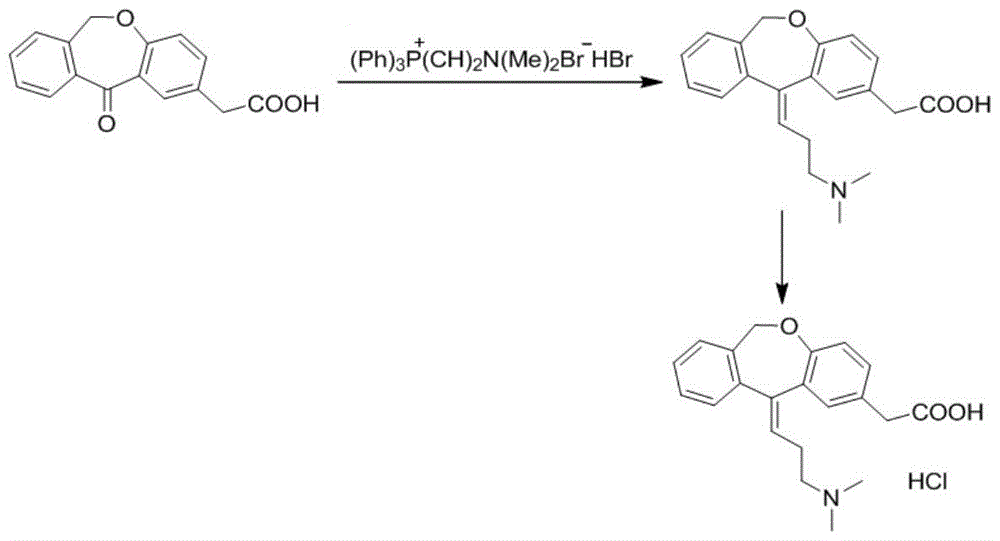
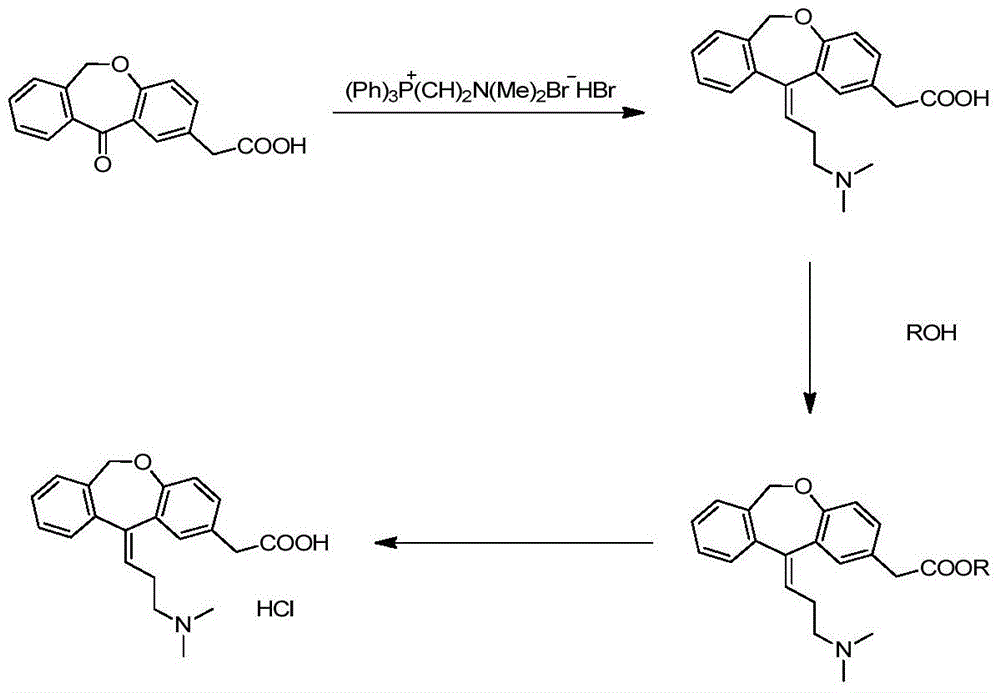
Preparation technique of olopatadine hydrochloride
A technology for the preparation of olopatadine hydrochloride and its preparation technology, which is applied in the field of preparation technology of olopatadine hydrochloride, can solve the problems that it is not suitable for industrial production, the proportion of Z-type products is small, and there are many by-products, and the synthesis steps are short, Strong nucleophilicity and the effect of improving the total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the preparation of isoket acid
[0047] Add 15.11g of p-hydroxyphenylacetic acid (100mmol), 4g of sodium hydroxide (100mmol), and 100ml of ethanol to the reaction flask, stir at room temperature for 1 hour, add 17.06g of 2-(chloromethyl)benzoic acid (100mmol), and heat up to reflux reaction , after the end of the thin-layer monitoring reaction, the solvent was distilled off under reduced pressure, dissolved in 200ml of dichloromethane, washed with water, dried with anhydrous magnesium sulfate, concentrated, added 1% Eaton reagent (mass concentration) 400ml, heated to 80 ° C, stirred for 2 Hours, after the reaction was completed, a saturated sodium carbonate solution was added, suction filtered, and recrystallized from ethanol to obtain 19.53 g of isoket acid (72.8 mmol), with a yield of 72.8% and a HPLC purity of 99.8%.
Embodiment 2
[0048] Embodiment 2: the preparation of isoket acid
[0049]Add 30.23g of p-hydroxyphenylacetic acid (200mmol), 31.80g of sodium carbonate (300mmol), 120ml of isopropanol in the reaction flask, stir at room temperature for 1 hour, add 17.06g of 2-(chloromethyl)benzoic acid (100mmol), and heat up Reflux reaction, thin-layer monitoring After the reaction is over, the solvent is distilled off under reduced pressure, dissolved in 200ml of dichloromethane, washed with water, dried, concentrated, 100ml of 10% Eaton reagent (mass concentration) is added, the temperature is raised to 80°C, stirred for 2 hours, and the reaction After completion, add saturated sodium carbonate solution, filter with suction, and recrystallize from ethanol to obtain 20.52 g of isoket acid (76.5 mmol), with a yield of 76.5% and a purity of 99.3% by HPLC.
Embodiment 3
[0050] Embodiment 3: the preparation of isoxacic acid
[0051] Add 22.67g of p-hydroxyphenylacetic acid (150mmol), 11.22g of potassium hydroxide (200mmol), and 90ml of methanol into the reaction flask, stir at room temperature for 1 hour, add 17.06g of 2-(chloromethyl)benzoic acid (100mmol), and heat up to reflux Reaction, thin-layer monitoring After the reaction is over, distill the solvent off under reduced pressure, add 200ml of dichloromethane to dissolve, wash with water, dry, concentrate, add 200ml of 7.5% Eaton reagent (mass concentration), heat up to 80°C, stir for 2 hours, and the reaction is complete Afterwards, saturated sodium carbonate solution was added, suction filtered, and recrystallized from ethanol to obtain 21.27 g of isoket acid (79.3 mmol), with a yield of 79.3% and a HPLC purity of 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com