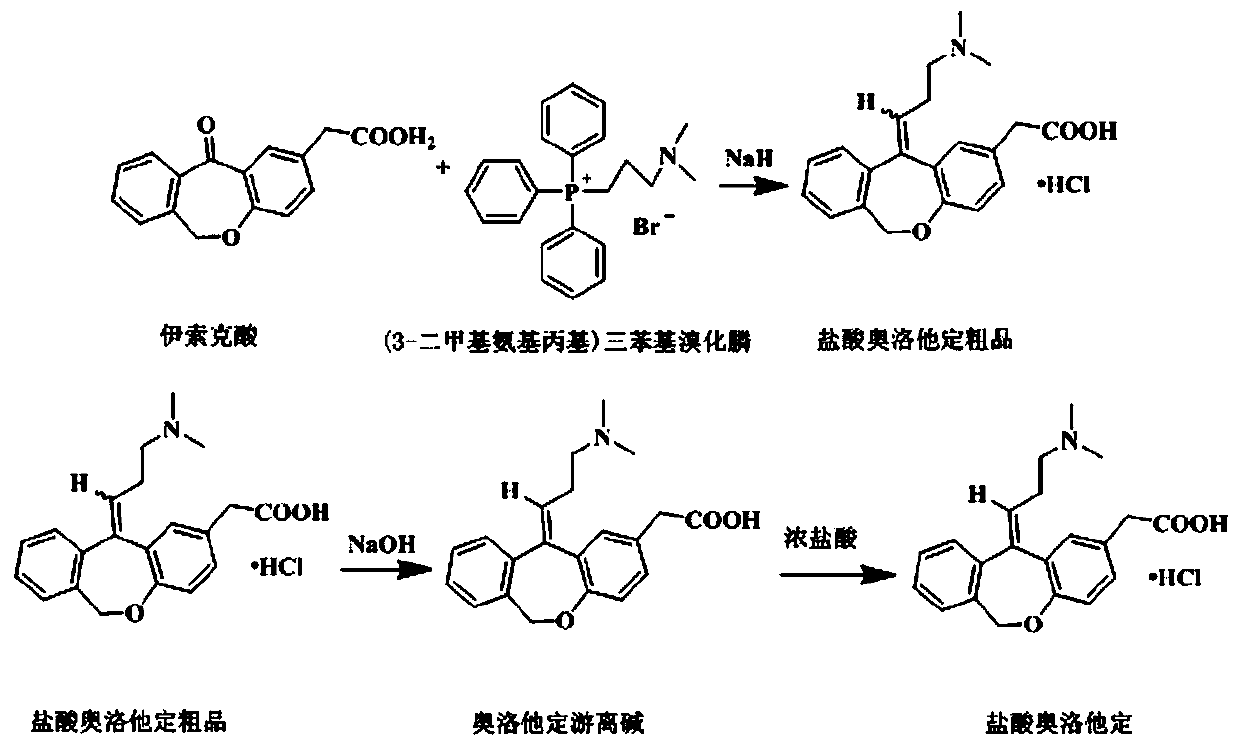
Preparation method of olopatadine hydrochloride
A technology of olopatadine hydrochloride and concentrated hydrochloric acid, which is applied in organic chemistry methods, organic chemistry, etc., can solve problems such as complex reaction routes, achieve high yield, ensure the same quality and yield, and optimize the effect of reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032](1) Add 200g (3-dimethylaminopropyl)triphenylphosphine bromide to the three-necked flask, then add 400g of anhydrous tetrahydrofuran, start stirring, control the temperature at 20~22°C, add 40g of NaH, and raise the temperature after adding To reflux, react for 1h. Then add 100g of isoket acid, reflux and stir for 2 hours, then cool down to 0~15°C, slowly add 30g of anhydrous methanol, then add 400g of tetrahydrofuran aqueous solution with a mass concentration of 50%, and finally add 1600g of water to quench the reaction. The reaction solution was adjusted to PH=6±0.2 with concentrated hydrochloric acid, then distilled to dryness under reduced pressure, and evaporated to dryness to obtain a solid. The solid was dissolved in 1400g of acetone, started stirring, and 40g of concentrated hydrochloric acid was added to precipitate a white solid. After the dropwise addition, the temperature was lowered to 10-15°C and stirred for another 10 hours. Suction filtration to obtain a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com