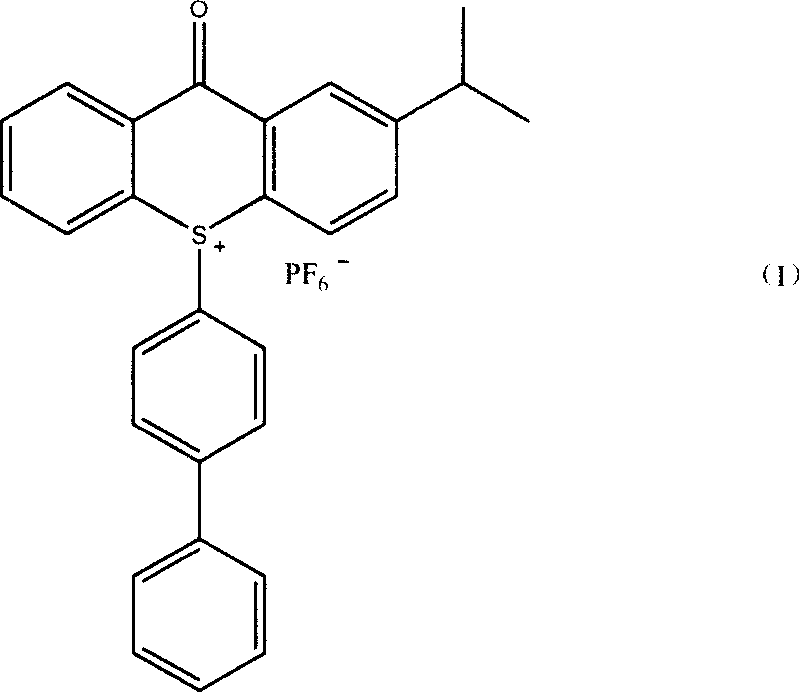
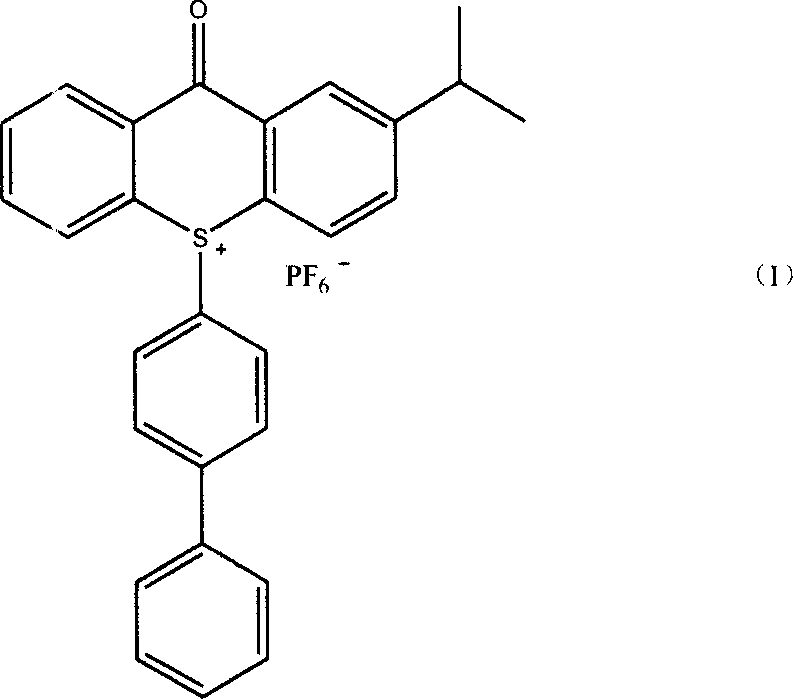
Method of producing 10-(4-xenyl)-2-isopropyl thioxanthone sulfur onium phosphorofluoric acid salt
A technology of isopropylthioxanthone and biphenyl is applied in the field of preparation of sulfonium salt compounds, and can solve the problems of poor product quality and high cost of production raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In a 1000 ml four-necked flask, add 60.0 grams (0.24 moles) of 2-ITX (2-isopropylthioxanthone), 450 milliliters of acetonitrile and 100 milliliters of water, 18.0 grams of cerium ammonium nitrate (0.0328 moles), 10.0 grams 48% hydrobromic acid solution, after stirring evenly, keep the temperature at 25-30°C, add 200 grams (0.24 moles) of 10% sodium hypochlorite solution dropwise from the dropping funnel, stir for 24 hours and take a sample analysis, the content of raw material 2-ITX was 1.1%. Add 400 ml of water to the reaction solution, stir for 1 hour, then filter with suction, wash the filter cake with 100 ml of water, and dry to obtain 56.1 g of light yellow oxidation product. The purity by HPLC analysis was 97.4%, and the yield was 88.4%.
Embodiment 2
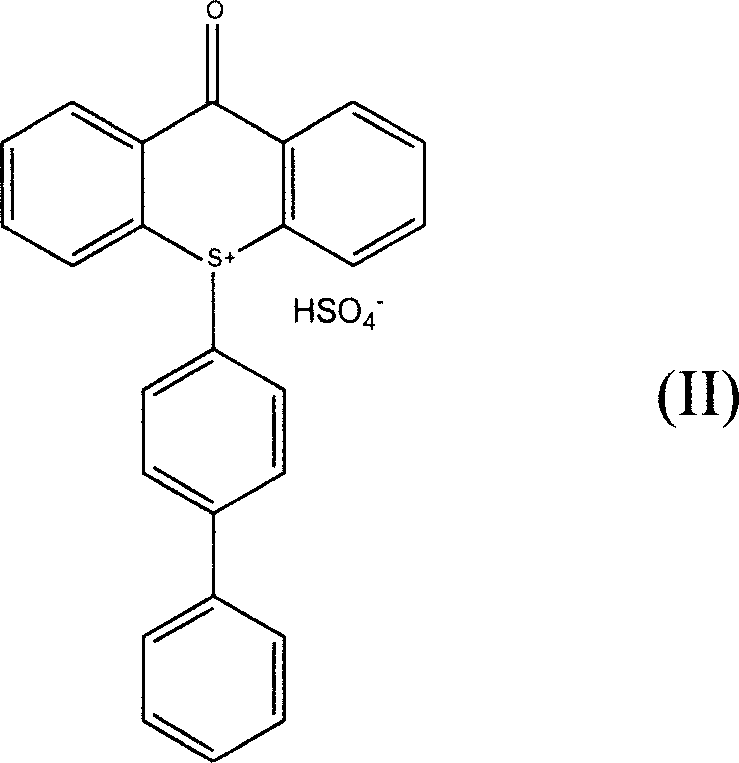
[0026] 20.5 grams (0.076 moles) of 2-ITX oxidation product (gained in Example 1) and 18.0 grams (0.117 moles) of biphenyl, 70 milliliters of acetic acid, 70 milliliters of acetic anhydride, 18 milliliters of dichloromethane, stirred in 250 milliliters four-necked flask Dissolve, then place in a low-temperature bath to cool down to below 15°C, add 26 ml of concentrated sulfuric acid dropwise, and keep the temperature of the reaction solution not higher than 15°C. After the dropwise addition, the mixture was stirred at 15°C for 2 hours. The reaction solution was transferred to a 2500 ml reaction bottle, 1000 ml of water and 1000 ml of dichloromethane were added, and after stirring for 30 minutes, the layers were separated in a separatory funnel, and the lower layer of dichloromethane solution was separated. Dichloromethane was evaporated to dryness on a rotary evaporator to obtain the bisulfate intermediate.
[0027] This intermediate was dissolved in 150 ml of methanol, and 25...
Embodiment 3
[0032]20.5 grams (0.076 moles) of 2-ITX oxidation product (gained in Example 1) and 18.0 grams (0.117 moles) of biphenyl, 70 milliliters of acetic acid, 70 milliliters of acetic anhydride, 18 milliliters of dichloromethane, stirred in 250 milliliters four-necked flask Dissolve, then place in a low-temperature bath to cool down to below 15°C, add 26 ml of concentrated sulfuric acid dropwise, and keep the temperature of the reaction solution not higher than 15°C. After the dropwise addition, the mixture was stirred at 15°C for 2 hours. Add 1000 milliliters of water and 1000 milliliters of dichloromethane, stir, and then transfer the reaction solution to a 2500 milliliter reaction flask. After 30 minutes, put in a separatory funnel to separate layers and separate the lower layer of dichloromethane solution. Dichloromethane was evaporated to dryness on a rotary evaporator to obtain the bisulfate intermediate.
[0033] This intermediate was dissolved in 150 ml of ethanol, and 250 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com