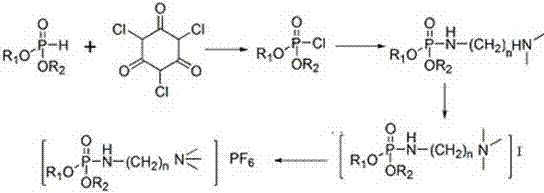
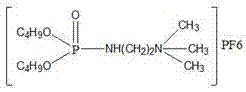Phosphate ionic liquids as well as synthetic method and application thereof
A technology of phosphate esters and ionic liquids, which is applied in chemical instruments and methods, liquid solution solvent extraction, compounds of group 5/15 elements of the periodic table, etc., can solve the problem of strong corrosion of extraction equipment and high concentration of tributyl phosphate , organic phase dissolution loss and other problems, to achieve the effect of favorable promotion and industrial production, feasible process, and reduced viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of dibutyl (2-(trimethyl-amino) ethyl) phosphonic acid hexafluorophosphate, the structural formula is as follows:
[0033]
[0034] Add 58.28g of dibutyl phosphite, 23.25g of trichloroisocyanuric acid and 270mL of acetonitrile into a 500mL three-necked flask, place the system on a constant temperature stirrer, react at room temperature, and stop the reaction within 15 minutes.
[0035] After suction filtration, the filter cake was washed three times with 50 mL of acetonitrile to obtain 65.12 g of the product with a yield of 95%.
[0036] Add 57.08g of phosphorus oxychloride, 180mL of 1,2-dichloroethane and 25.25g of triethylamine into a 500mL three-necked flask. The system is lowered to below zero in the ice-salt bath, and 60ml containing 24.38g of N,N - 1,2-dichloroethane solution of dimethylethylenediamine, the rate of addition is controlled to ensure that the reaction temperature is maintained within 10°C. After the dropwise addition, the reaction syste...
Embodiment 2
[0042] Synthesis of diethyl (2-(trimethyl-amino)butyl) phosphonic acid hexafluorophosphate, the structural formula is as follows:
[0043]
[0044] Add 41.45g of diethyl phosphite, 23.25g of trichloroisocyanuric acid and 240mL of acetonitrile into a 500mL three-neck flask, place the system on a constant temperature stirrer, react at room temperature, and stop the reaction within 15 minutes.
[0045] After suction filtration, the filter cake was washed three times with 45 mL of acetonitrile to obtain 47.87 g of the product with a yield of 93.5%.
[0046] Add 43.17g of phosphorus oxychloride, 150mL of 1,2-dichloroethane and 25.25g of triethylamine into a 500mL three-necked flask. The system is lowered to below zero in an ice-salt bath, and 90ml containing 29.75g of N,N - 1,2-dichloroethane solution of dimethylethylenediamine, the rate of addition is controlled to ensure that the reaction temperature is maintained within 10°C. After the dropwise addition, the reaction system ...
Embodiment 3
[0052] Synthesis of diethyl(2-(trimethyl-amino)propyl)phosphonic acid hexafluorophosphate, the structural formula is as follows:
[0053]
[0054] Add 57.98g of dibutyl phosphite, 22.95g of trichloroisocyanuric acid and 270mL of acetonitrile into a 500mL three-necked flask, place the system on a constant temperature stirrer, react at room temperature, and stop the reaction within 15 minutes.
[0055] After suction filtration, the filter cake was washed three times with 50 mL of acetonitrile to obtain 64.78 g of the product with a yield of 94.7%.
[0056] Add 56.98g of phosphorus oxychloride, 180mL of 1,2-dichloroethane and 25.31g of triethylamine into a 500mL three-necked flask, and the system is lowered to below zero in an ice-salt bath, and 25.56g of N,N- 60mL 1,2-dichloroethane solution of dimethylpropanediamine, the acceleration is controlled to ensure that the reaction temperature is maintained within 10°C. After the dropwise addition, the reaction system was transfer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com