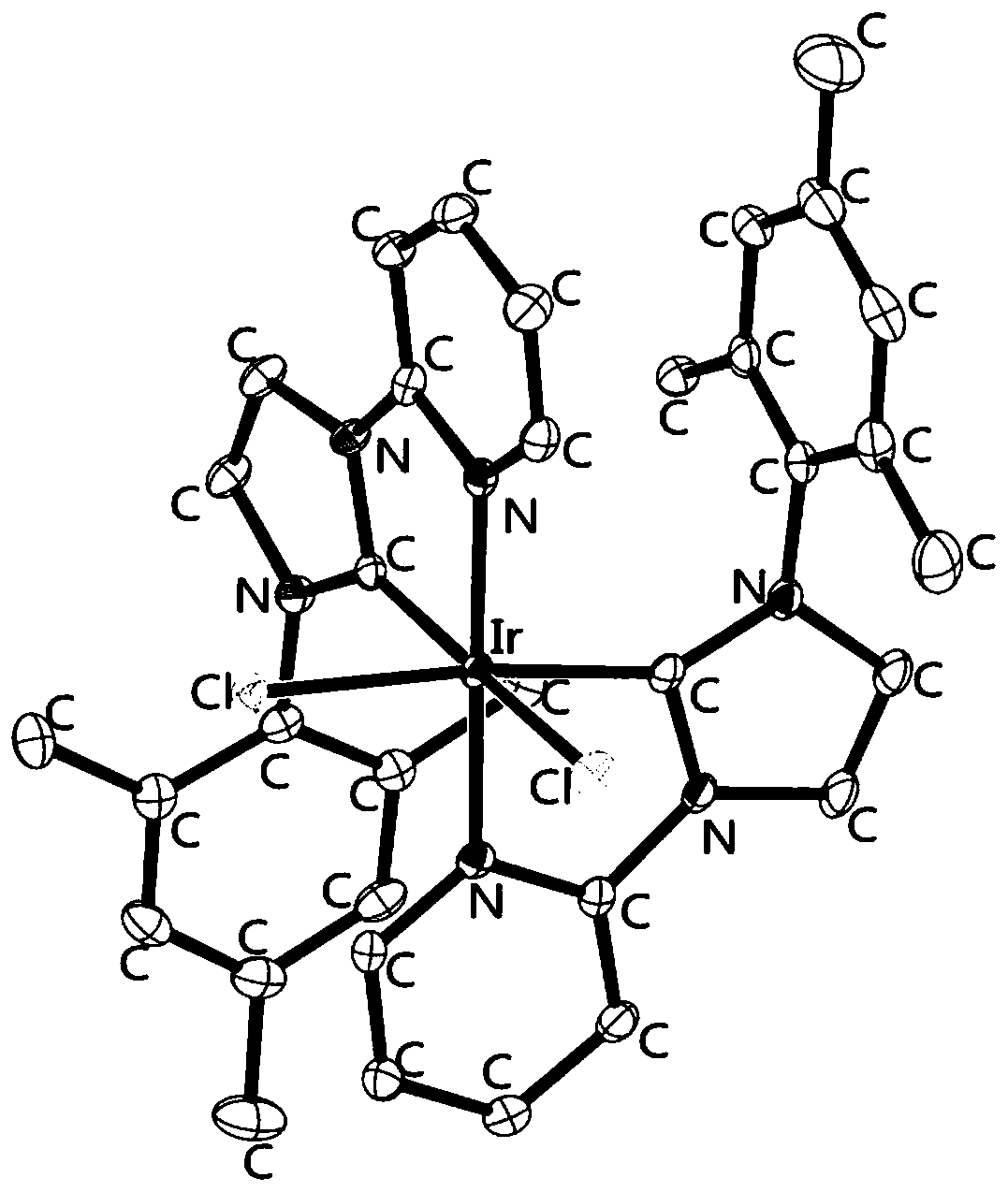
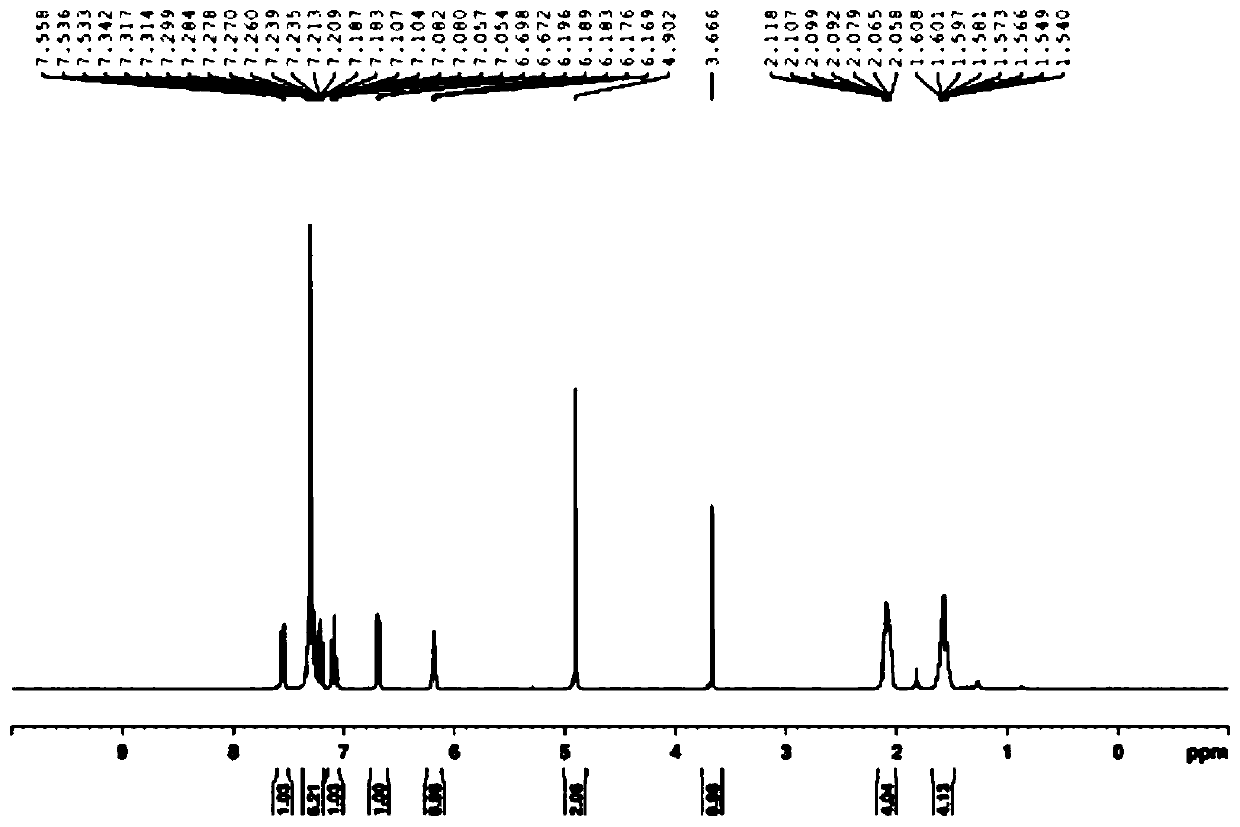
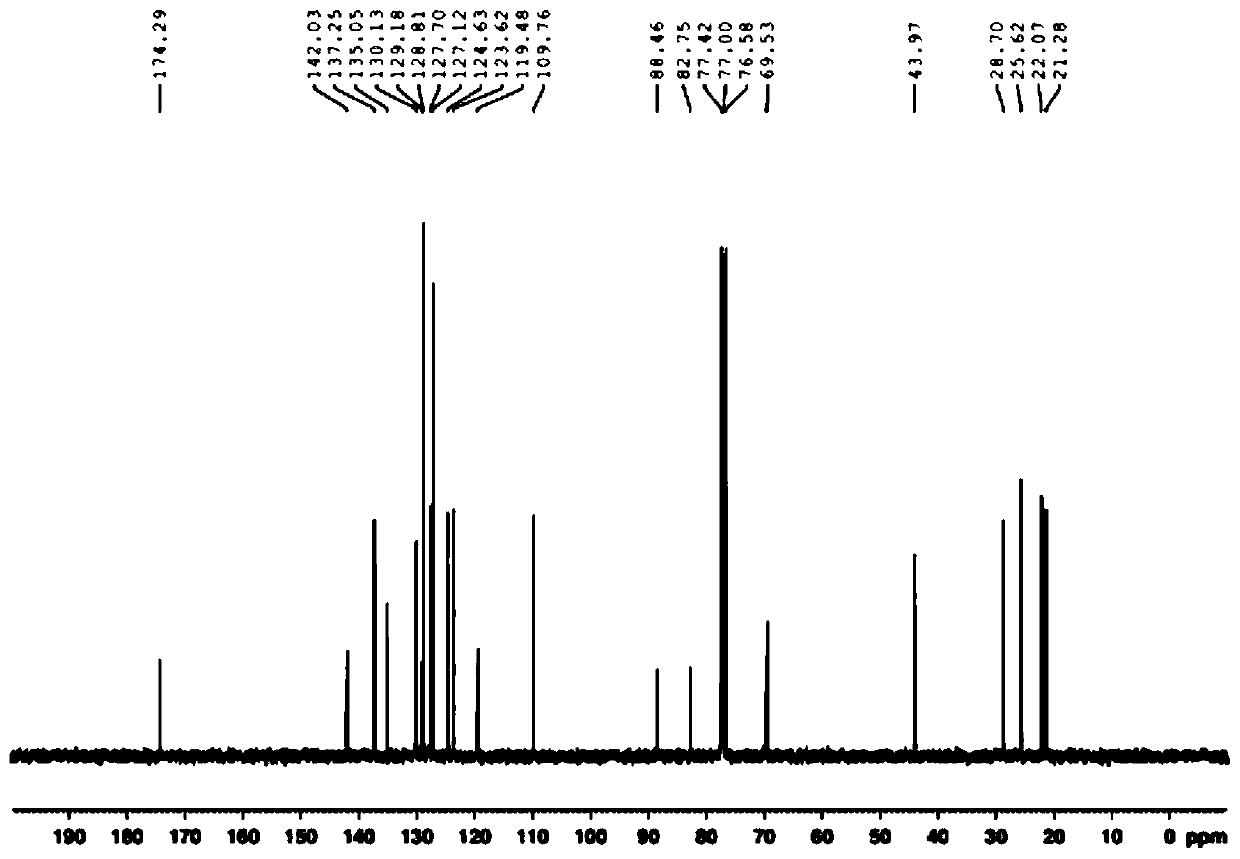
Metal iridium-carbene complex with photocatalytic performance as well as preparation method and application thereof
A carbene complex, metal iridium technology, applied in indium organic compounds, platinum group organic compounds, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problems of difficult industrial production, harsh reaction conditions, etc. Catalytic effect, high photocatalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] Another aspect of the embodiments of the present invention provides a method for preparing the above metal iridium-carbene complex with photocatalytic properties, comprising the following steps:
[0040] (1) Synthesis of carbene precursor pyridine-imidazolium bromide:
[0041]
[0042] Mix N-(2,4,6-trimethylphenyl)imidazole and 2-bromopyridine as shown in the structure of formula I, seal, heat up and stir the reaction under solvent-free conditions, cool to room temperature after the reaction, and obtain The first crude product, the first crude product is taken out and washed with an ether solvent and suction filtered to obtain the carbene precursor pyridine-imidazolium bromide shown in the structure of formula II;
[0043] (2) Synthesis of metal iridium-carbene complexes:
[0044]
[0045] i) Dissolve the carbene precursor pyridine-imidazolium bromide salt prepared in step (1) in a halogenated hydrocarbon solvent, then add silver oxide solids, and react for 1 h a...
Embodiment 1
[0059] (1) Synthesis of carbene precursor pyridine-imidazolium bromide:
[0060]
[0061] In a high-temperature reaction tube equipped with a magnetic stirring bar, add 18.6 grams (100 mmol) of N-(2,4,6-trimethylphenyl) imidazole solid as shown in the structure of formula I, and then add 16.5 grams (105 mmol) of 2-bromopyridine, mix well, then seal the tube, heat up to 180°C, melt and stir for 18 hours under solvent-free conditions, cool to room temperature after the reaction, and obtain the first crude product, scrape the first crude product Take it out and put it into a centrifuge tube, add diethyl ether and shake and wash the first crude product 5 times (30mL×5 times each time), filter with suction, and obtain 30.9 grams of milky white powder after drying, which is the carbene precursor shown in the structure of formula II Pyridine-imidazolium bromide, yield 90wt%;
[0062] The reaction of step (1) is a solvent-free reaction. At high temperature, the N-(2,4,6-trimethylp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com