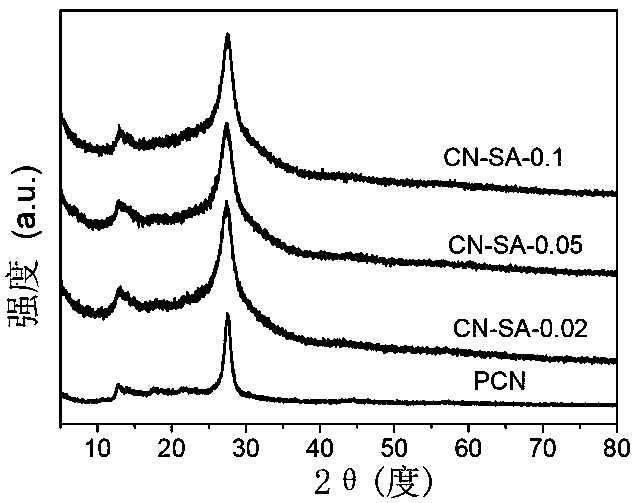
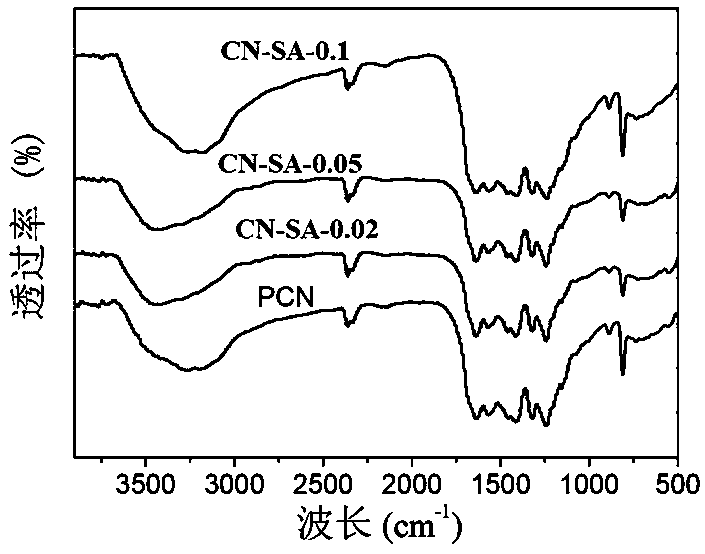
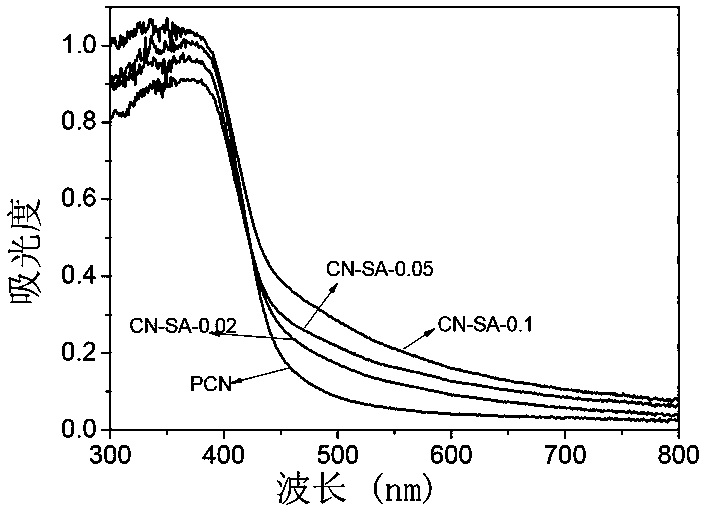
Modified graphite phase carbon nitride photocatalyst as well as preparation method and application thereof
A carbon nitride photocatalyst technology, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problem of insufficient catalytic degradation performance, limit the wide application of copolymerization modification, and photogenerated carriers Easy to recombine and other problems, to improve the utilization rate of electrons and photocatalytic efficiency, increase the number of reactive active sites, and enhance the effect of surface mass transfer process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A modified graphite phase carbon nitride photocatalyst is prepared by calcining urea and salicylic acid as raw materials. The mass ratio of urea and salicylic acid is 1:0.002. The preparation method comprises the following steps:
[0034] Take 10g of urea and 0.02g of salicylic acid, grind them, dissolve them in 20mL of water, stir for 2h, and dry them on an electric furnace (at a temperature of 80°C for 10h) to obtain a precursor. The precursor was placed in a muffle furnace, heated to 550°C at a heating rate of 15°C / min, and kept at 550°C for 2 hours to calcine the precursor. Copolymerization was achieved through calcination to obtain a modified graphite phase carbon nitride light. Catalyst, take out the block after natural cooling, and grind to obtain a modified graphite phase carbon nitride photocatalyst, named CN-SA-0.02.
Embodiment 2
[0036] A modified graphite-phase carbon nitride photocatalyst is prepared by calcining urea and salicylic acid as raw materials. The mass ratio of urea and salicylic acid is 1:0.005. The preparation method specifically includes the following steps:
[0037] Take 10g of urea and 0.05g of salicylic acid, grind them, dissolve them in 20mL of water, stir for 2h, and dry them on an electric furnace to obtain a precursor. The precursor was placed in a muffle furnace, heated to 550°C at a heating rate of 15°C / min, and kept at 550°C for 2 hours to calcine the precursor. Copolymerization was achieved through calcination to obtain a modified graphite phase carbon nitride light. Catalyst, take out the block after natural cooling, and grind to obtain a modified graphite phase carbon nitride photocatalyst, named CN-SA-0.05.
Embodiment 3
[0039] A modified graphite phase carbon nitride photocatalyst is prepared by calcining urea and salicylic acid as raw materials. The mass ratio of urea and salicylic acid is 1: 0.01. The preparation method comprises the following steps:
[0040] Take 10g of urea and 0.1g of salicylic acid, grind them, dissolve them in 20mL of water, stir for 2h, and dry them on an electric furnace to obtain a precursor. The precursor was placed in a muffle furnace, heated to 550°C at a heating rate of 15°C / min, and kept at 550°C for 2 hours to calcine the precursor, and achieve copolymerization through calcination to obtain a modified graphite phase carbon nitride photocatalyst , after natural cooling, the block was taken out and ground to obtain a modified graphite phase carbon nitride photocatalyst, which was named CN-SA-0.1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com