Method for detecting atomoxetine hydrochloride enantiomer by reversed-phase liquid chromatography
A technology of atomoxetine hydrochloride and reversed-phase liquid chromatography, which is applied in the field of drug analysis, can solve the problems of unfavorable enterprise product quality control, complicated operation, high requirements, etc., and achieves short detection time, simple operation process, high performance The effect of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The screening of embodiment 1 stationary phase
[0040] Chromatography instrument: Dion U-3000 high performance liquid chromatography
[0041] Chromatographic column: Chiralpak AD-RH, Chiralpak AS-RH, Chiralcel OD-RH and Chiralcel OJ-RH (150mm*4.6mm, 5μm)
[0042] Detector and wavelength: UV-225
[0043] Flow rate: 0.7ml / min
[0044] Injection volume: 10μl
[0045] Column temperature: 30°C
[0046] Mobile phase: 0.1mol / L potassium hexafluorophosphate solution (add 0.1% diethylamine, adjust pH to 4.5 with phosphoric acid)-acetonitrile (72:28, v / v)
[0047] Diluent: mobile phase.
[0048] Preparation of sensitivity solution: Accurately weigh the enantiomers of atomoxetine hydrochloride, and prepare a solution with a concentration of 0.015 μg / ml with diluent.
[0049] System suitability solution: Take an appropriate amount of atomoxetine hydrochloride racemate, add mobile phase to dissolve and quantitatively dilute to make a solution containing about 0.02mg per 1ml, ...
Embodiment 2
[0055] The screening of embodiment 2 mobile phases
[0056] (1) Detection effect test of different mobile phase ratios
[0057] Mobile phase one: Potassium hexafluorophosphate solution-acetonitrile (77:23, v / v)
[0058] Mobile phase two: Potassium hexafluorophosphate solution-acetonitrile (72:28, v / v)
[0059] Mobile phase three: Potassium hexafluorophosphate solution-acetonitrile (67:33, v / v)
[0060] Chromatography instrument: Dion U-3000 high performance liquid chromatography
[0061] Chromatographic column: Chiralcel OJ-RH (150mm*4.6mm, 5μm)
[0062] Detector and wavelength: UV-225
[0063]Flow rate: 0.7ml / min
[0064] Injection volume: 10μl
[0065] Column temperature: 30°C
[0066] Diluent: mobile phase
[0067] Table 2 Mobile phase ratio screening for enantiomer detection
[0068]
[0069] The sensitivity and resolution results of different mobile phases are shown in Table 2.
[0070] Conclusion: When the mobile phase ratio is 72:28, the S / N value is the hi...
Embodiment 3
[0102] Embodiment 3 methodological investigation
[0103] (1) System suitability
[0104] Chromatography instrument: Dion U-3000 high performance liquid chromatography
[0105] Chromatographic column: Chiralcel OJ-RH (150mm*4.6mm, 5μm)
[0106] Detector and wavelength: UV-225
[0107] Flow rate: 0.7ml / min
[0108] Injection volume: 10μl
[0109] Column temperature: 30°C
[0110] Mobile phase: 0.1mol / L potassium hexafluorophosphate solution (add 0.1% diethylamine, adjust pH to 4.5 with phosphoric acid)-acetonitrile (72:28, v / v)
[0111] Diluent: mobile phase.
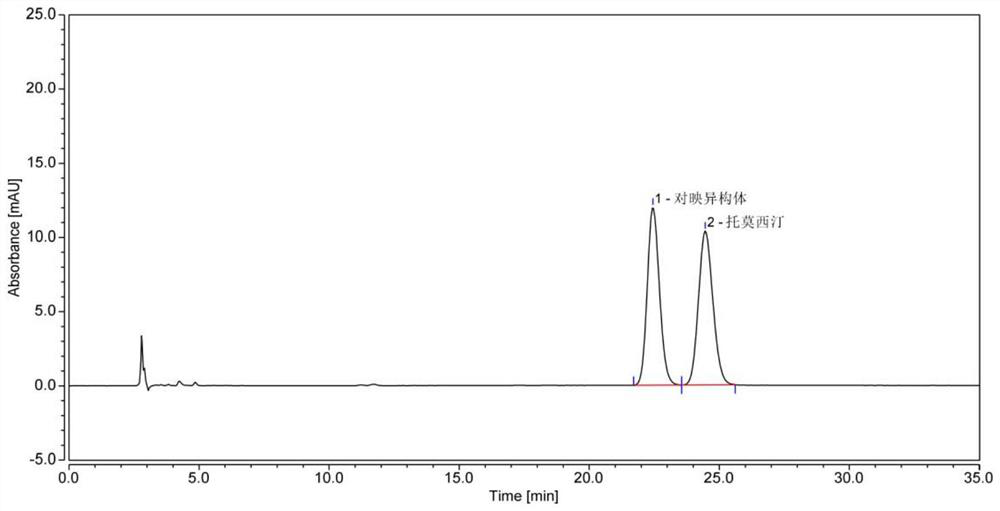
[0112] System suitability solution: Take an appropriate amount of atomoxetine hydrochloride racemate, add mobile phase to dissolve and quantitatively dilute to make a solution containing about 0.02mg per 1ml, as the system suitability solution. Inject 10 μl, the chromatographic results are as follows figure 1 shown.
[0113] Conclusion: It can be seen that in the system suitability solution chromatogram, the pea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com