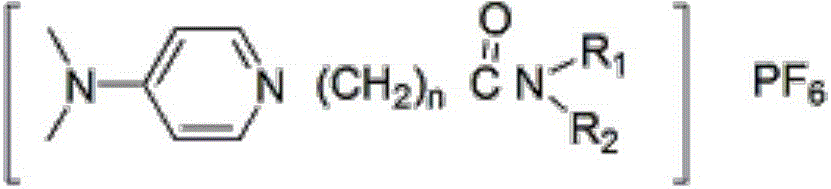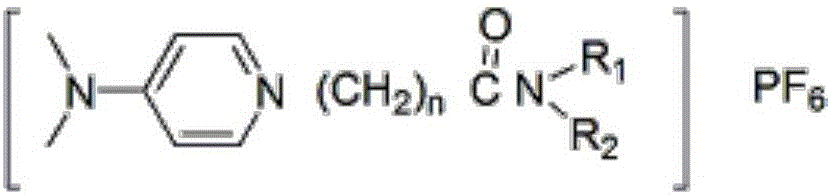Functionalized ion liquid for lithium extraction, and synthesis method thereof
A technology of ionic liquid and synthesis method, applied in the direction of organic chemistry, improvement of process efficiency, etc., can solve the problem of high corrosion of equipment, and achieve the effect of reducing corrosion, feasible process and easy control of reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Synthesis of 4-N, N, dimethyl-N'-bis(2-ethylhexyl)carbonylethylpyridine hexafluorophosphate:
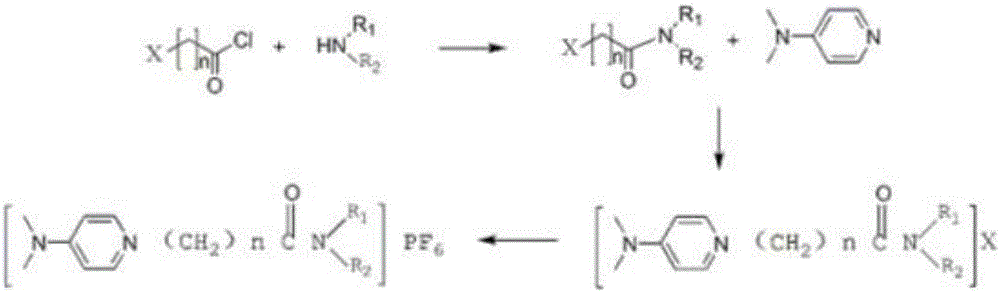
[0044] Add 26.65g of diisooctylamine, 80ml of 1,2-dichloroethane and 12.12g of triethylamine into a 500ml three-necked flask, place the system in an ice-salt bath and drop it below zero, and add 11.2g of chlorine Acetyl chloride in 35 ml of 1,2-dichloroethane solution, the rate of addition was controlled to ensure that the reaction temperature was maintained within 10°C. After the dropwise addition, the reaction system was transferred to room temperature and reacted for 3 h.
[0045] Add 100ml of 1M hydrochloric acid solution dropwise to quench the experiment, let stand, separate the phases, and keep the organic phase; the organic phase was washed with 100ml of 1M sodium hydroxide solution, 100ml of water, and 100ml of saturated brine, and finally dried over anhydrous sodium sulfate. After suction filtration and spin-drying, the obtained product was dried in a vacuum drying o...
Embodiment 2
[0049] Synthesis of 4-N, N, dimethyl-N'-diethylcarbonylpropylpyridine hexafluorophosphate:
[0050] Add 25.42g of diethylamine, 70ml of 1,2-dichloroethane and 22.26g of triethylamine into a 500ml three-necked flask, place the system in an ice-salt bath and drop it below zero, and add 16.09g of 3- 48 ml of 1,2-dichloroethane solution of chloropropionyl chloride, the rate of addition is controlled to ensure that the reaction temperature is maintained within 10°C. After the dropwise addition, the reaction system was transferred to room temperature and reacted for 3 h.
[0051] Add 150ml of 1M hydrochloric acid solution dropwise to quench the experiment, let it stand, separate the phases, and keep the organic phase; the organic phase was successively washed with 150ml of 1M sodium hydroxide solution, 150ml of water, and 150ml of saturated saline, and finally dried with anhydrous sodium sulfate. After suction filtration and spin-drying, the obtained product was dried in a vacuum dry...
Embodiment 3
[0055] Synthesis of 4-N, N, dimethyl-N'-bis(2-ethylhexyl)carbonylpropylpyridine hexafluorophosphate:
[0056] Add 26.35g of diisooctylamine, 80ml of 1,2-dichloroethane and 12.35g of triethylamine into a 500ml three-necked flask, place the system in an ice-salt bath and drop it below zero, and add 12.73g of 3 - 35 ml of 1,2-dichloroethane solution of chloropropionyl chloride, the rate of addition is controlled to ensure that the reaction temperature is maintained within 10°C. After the dropwise addition, the reaction system was transferred to room temperature and reacted for 3 h.
[0057] Add 110ml of 1M hydrochloric acid solution dropwise to quench the experiment, let it stand, separate the phases, and keep the organic phase; the organic phase was successively washed with 110ml of 1M sodium hydroxide solution, 110ml of water, and 110ml of saturated brine, and finally dried over anhydrous sodium sulfate. After suction filtration and spin-drying, the obtained product was dried ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com