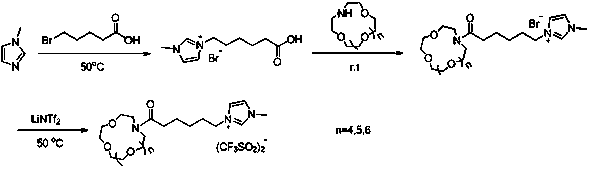
Synthesis method of aza-crown ether functionalized ionic liquid
An azacrown ether, ionic liquid technology, applied in the fields of organic synthesis and supramolecules, to achieve the effect of improving stability, optimizing synthesis process and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Step 1: In a 100 mL single-necked round bottom flask, add 6-bromohexanoic acid (0.290 g, 1.5 mmol), 1-methylimidazole (0.123 g, 1.5 mmol), and 30 mL of acetone, and install Spherical condenser, stirred and refluxed for 36 h, after the reaction was completed, the acetone was removed by rotary evaporation to generate 1-carboxy-3-methylimidazolium bromide;
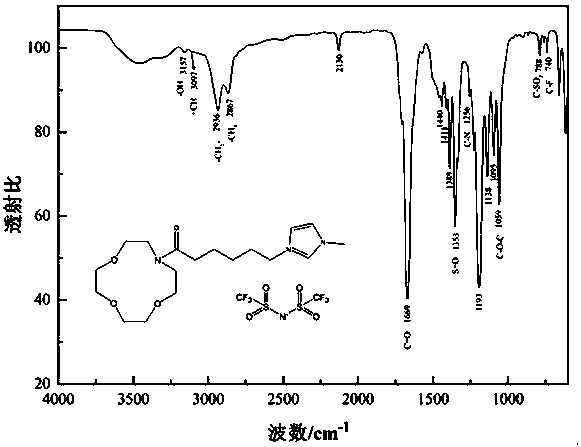
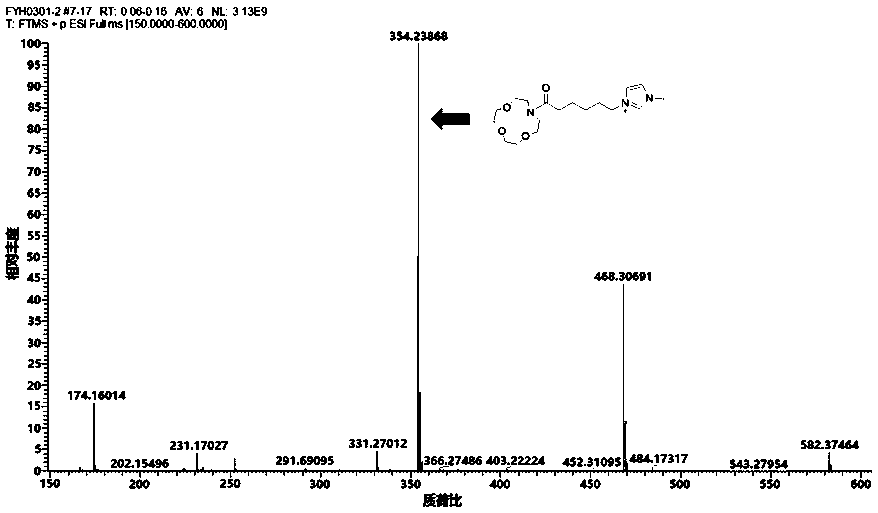
[0023] Step 2: Dissolve 1-carboxy-3-methylimidazolium bromide obtained in the previous step in an appropriate amount of N,N-dimethylformamide, add 1-(3-dimethylaminopropyl)-3-ethyl carbon Diimine hydrochloride (0.287 g, 1.5 mmol) and 1-hydroxybenzotriazole (0.202 g, 1.5 mmol) were stirred at 0 °C for 0.5 h to activate the carboxyl group, and then aza 12-crown-4 ether ( 0.263 g, 1.5 mmol) and triethylamine (0.152 g, 1.5 mmol) were stirred at room temperature for 24 h. After the reaction, the reactants were poured into 100 mL of water, extracted with dichloromethane (20 mL×3), the organic phases were combined, and washe...
Embodiment 2
[0026] Step 1: In a 100 mL single-necked round bottom flask, add 6-bromohexanoic acid (0.290 g, 1.5 mmol), 1-methylimidazole (0.123 g, 1.5 mmol), and 30 mL of acetone, and install Spherical condenser, stirred and refluxed for 36 h, after the reaction was completed, the acetone was removed by rotary evaporation to generate 1-carboxy-3-methylimidazolium bromide;
[0027] Step 2: Dissolve 1-carboxy-3-methylimidazolium bromide obtained in the previous step in an appropriate amount of N,N-dimethylformamide, add 1-(3-dimethylaminopropyl)-3-ethyl carbon Diimine hydrochloride (0.287 g, 1.5 mmol) and 1-hydroxybenzotriazole (0.202 g, 1.5 mmol) were stirred at 0 °C for 0.5 h to activate the carboxyl group, and then aza-15-crown-5 ether ( 0.328 g, 1.5 mmol) and triethylamine (0.152 g, 1.5 mmol) were stirred at room temperature for 24 h. After the reaction, the reactants were poured into 100 mL of water, extracted with dichloromethane (20 mL×3), the organic phases were combined, and washe...
Embodiment 3
[0030] Step 1: In a 100 mL single-necked round bottom flask, add 6-bromohexanoic acid (0.290 g, 1.5 mmol), 1-methylimidazole (0.123 g, 1.5 mmol), and 30 mL of acetone, and install Spherical condenser, stirred and refluxed for 36 h, after the reaction was completed, the acetone was removed by rotary evaporation to generate 1-carboxy-3-methylimidazolium bromide;
[0031] Step 2: Dissolve 1-carboxy-3-methylimidazolium bromide obtained in the previous step in an appropriate amount of N,N-dimethylformamide, add 1-(3-dimethylaminopropyl)-3-ethyl carbon Diimine hydrochloride (0.287 g, 1.5 mmol) and 1-hydroxybenzotriazole (0.202 g, 1.5 mmol) were stirred at 0 °C for 0.5 h to activate the carboxyl group, and then aza-18-crown-6 ether ( 0.395 g, 1.5 mmol) and triethylamine (0.152 g, 1.5 mmol) were stirred at room temperature for 24 h. After the reaction, the reactants were poured into 100 mL of water, extracted with dichloromethane (20 mL×3), the organic phases were combined, and washe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com