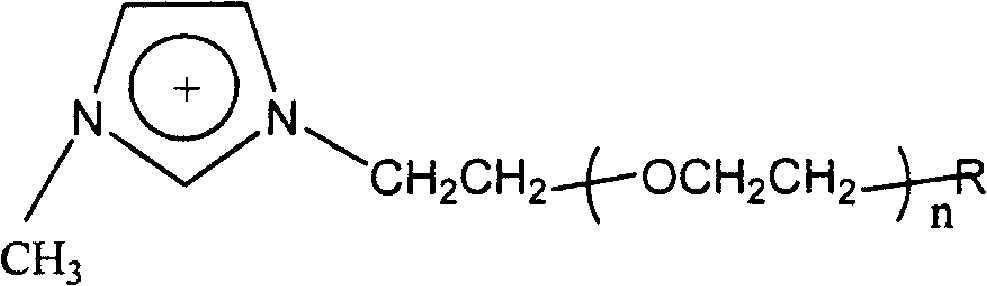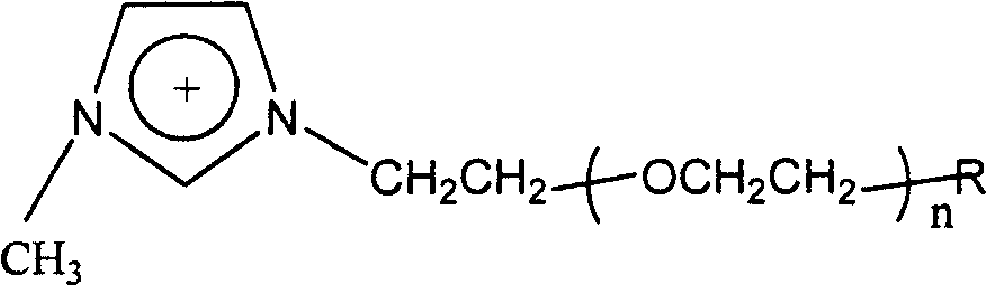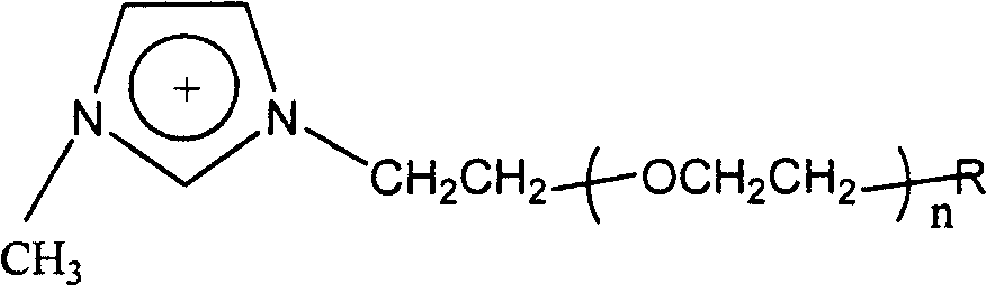Gel electrolyte for dye-sensitized nanocrystal solar cell
A gel electrolyte and solar cell technology, applied in the field of electrolyte material preparation, can solve problems such as dye degradation, complicated sealing process, and increased opportunities for photogenerated charge recombination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A gel electrolyte for a dye-sensitized nanocrystalline solar cell, the gel electrolyte includes an organic molten salt and a nano-tin oxide gel, and the volume ratio of the organic molten salt to the nano-tin oxide gel is 50:7; the organic molten salt The cation is the imidazolium cation substituted by low molecular weight polyethylene oxide, and the structure of the imidazolium cation is as follows:
[0027]
[0028] Among them, n is 9-14; R group is H, OH, OCH 3 non-polymerizable group or CH 2 = CHCOO-, CH 2 =CCH 3 COO-polymerizable group, the anion of the molten salt is fluoride ion, the molecular weight of the low molecular weight polyethylene oxide is 450; the conductivity of the gel electrolyte is 15.3mS·cm -1 .
Embodiment 2
[0030] A gel electrolyte for a dye-sensitized nanocrystalline solar cell, the gel electrolyte includes an organic molten salt and a nano-tin oxide gel, and the volume ratio of the organic molten salt to the nano-tin oxide gel is 5:1; the organic molten salt The cation is the imidazolium cation substituted by low molecular weight polyethylene oxide, and the structure of the imidazolium cation is as follows:
[0031]
[0032] Among them, n is 9-14; R group is H, OH, OCH 3 non-polymerizable group or CH 2 = CHCOO-, CH 2 =CCH 3 COO-polymerizable group, the anion of the molten salt is fluoride ion, the molecular weight of the low molecular weight polyethylene oxide is 450; the conductivity of the gel electrolyte is 16.5mS cm -1 .
Embodiment 3
[0034] A gel electrolyte for dye-sensitized nanocrystalline solar cells, the gel electrolyte contains organic molten salt, nano tin oxide gel and Al 2 o 3 Nano particle gel, organic molten salt, nano tin oxide gel, Al 2 o 3 The volume ratio of the nanoparticle gel is 50:15:1; the cation of the organic molten salt is a polyethylene oxide substituted imidazolium cation of low molecular weight, and the structure of the imidazolium cation is as follows:
[0035]
[0036] Among them, n is 9-14; R group is H, OH, OCH 3 non-polymerizable group or CH 2 = CHCOO-, CH 2 =CCH 3 COO-polymerizable group, the anion of the molten salt is fluoride ion; the molecular weight of the low molecular weight polyethylene oxide is 550; the conductivity of the gel electrolyte is 18.7mS·cm -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com