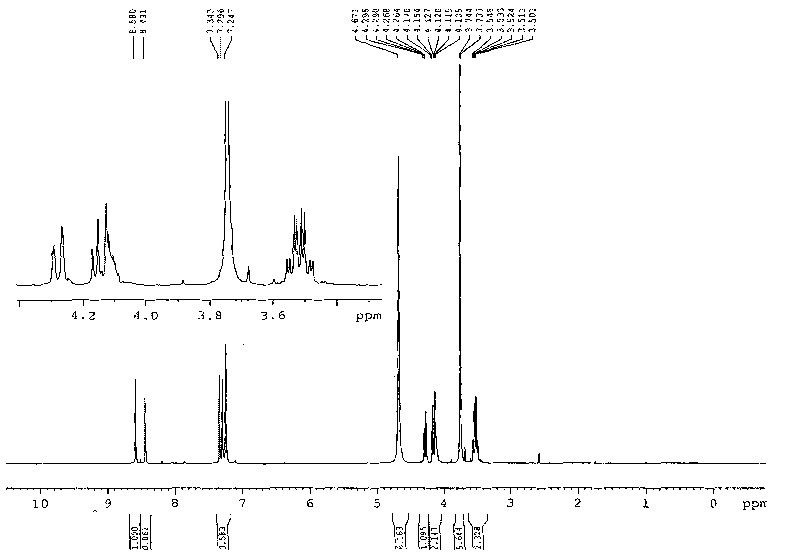
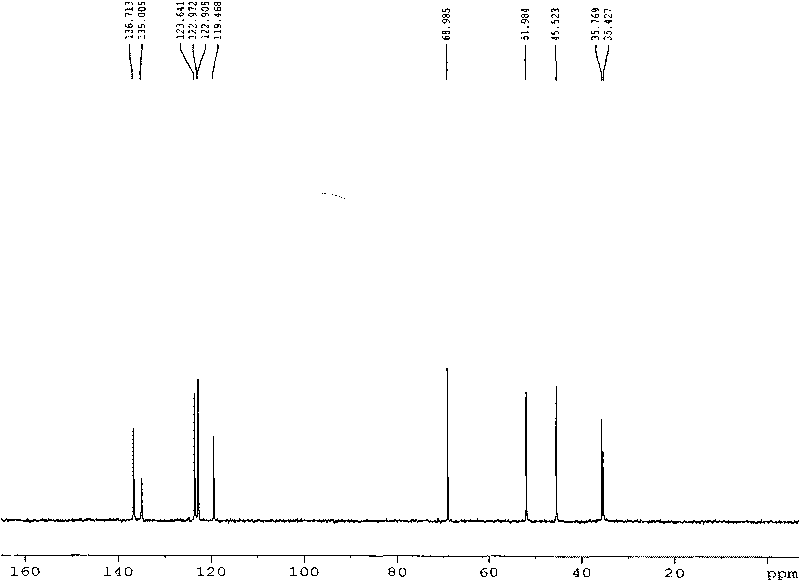
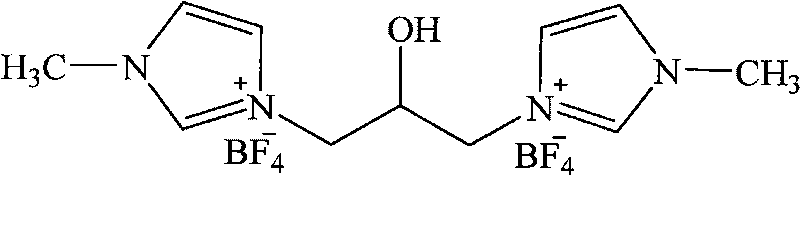
1, 3-di-(1-methylimidazole)-2-propanol tetrafluoroborate ion liquid and preparation method thereof
A technology of methyl imidazole tetrafluoroborate and propanol tetrafluoroborate, which is applied in 1 field and achieves the effects of convenient operation, good thermal stability and low vapor pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Synthesis of 1-(3-chloro-2-hydroxy-propyl)-3-alkylimidazolium tetrafluoroborate ionic liquid
[0026] Dissolve 20mL of 1-methylimidazole (10.3g, 0.25mol) in 50mL of ethanol, add 33.20mL of tetrafluoroboric acid (0.25mol, 48% aqueous solution) in batches under stirring at 0°C, and react for 60 minutes under the same conditions after the addition 19.6 mL of 3-chloro-propylene oxide (23.12 g, 0.25 mol) was added dropwise to the reaction solution under stirring condition. After the drying tube was equipped, the reaction bottle was placed in the water bath of the experimental ultrasonic cleaner, and reacted with ultrasonic radiation (power 300W, frequency 40KHz) at 20°C for 3h, and TLC detected that the reactant disappeared. The solvent was removed by rotary evaporation under reduced pressure at 60°C, and the colorless liquid was 51.18g of 1-(3-chloro-2-hydroxy-propyl)-3-methylimidazolium tetrafluoroborate ionic liquid after vacuum drying at 80°C for 12 hours , the yiel...
Embodiment 2
[0030] (1) Synthesis of 1-(3-chloro-2-hydroxy-propyl)-3-alkylimidazolium tetrafluoroborate ionic liquid
[0031] Dissolve 20mL of 1-methylimidazole (10.3g, 0.25mol) in 50mL of ethanol, add 33.20mL of tetrafluoroboric acid (0.25mol, 48% aqueous solution) in batches under stirring at 0°C, and react for 60 minutes under the same conditions after the addition 19.6 mL of 3-chloro-propylene oxide (23.12 g, 0.25 mol) was added dropwise to the reaction solution under stirring condition. After the drying tube was equipped, the reaction bottle was placed in the water bath of the ultrasonic cleaner used in the experiment, and reacted with ultrasonic radiation (power 300W, frequency 40KHz) at 30°C for 4h, and the reactant disappeared as detected by TLC. The solvent was removed by rotary evaporation under reduced pressure at 60°C, and the colorless liquid was 53.06g of 1-(3-chloro-2-hydroxy-propyl)-3-methylimidazolium tetrafluoroborate ionic liquid after vacuum drying at 80°C for 12 hours ...
Embodiment 3
[0035] (1) Synthesis of 1-(3-chloro-2-hydroxy-propyl)-3-alkylimidazolium tetrafluoroborate ionic liquid
[0036] Dissolve 20mL of 1-methylimidazole (10.3g, 0.25mol) in 50mL of ethanol, add 33.20mL of tetrafluoroboric acid (0.25mol, 48% aqueous solution) in batches under stirring at 0°C, and react for 60 minutes under the same conditions after the addition 19.6 mL of 3-chloro-propylene oxide (23.12 g, 0.25 mol) was added dropwise to the reaction solution under stirring condition. After the drying tube was equipped, the reaction bottle was placed in the water bath of the ultrasonic cleaner used in the experiment, and reacted under ultrasonic radiation (power 300W, frequency 40KHz) at 40°C for 6h, and the reactant disappeared as detected by TLC. The solvent was removed by rotary evaporation under reduced pressure at 60°C, and the colorless liquid was 55.68g of 1-(3-chloro-2-hydroxy-propyl)-3-methylimidazolium tetrafluoroborate ionic liquid after vacuum drying at 80°C for 12 hours...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com