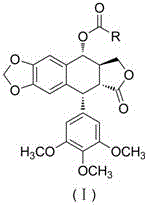
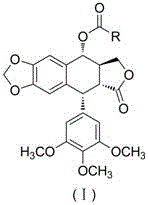
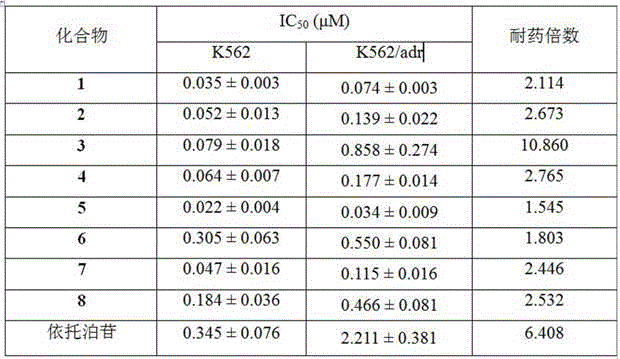
Heterocyclic aromatic acid ester type podophyllotoxin derivatives with anti-tumor activity as well as preparation method and application
A technology of anti-tumor activity and podophyllotoxin, applied in the direction of anti-tumor drugs, organic chemistry, drug combination, etc., can solve the problems of high toxicity and side effects, easy to produce drug resistance, etc., and achieve a strong inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of 4α-(thiazole-4-yl)-4-deoxy-podophyllotoxin (1)
[0026] Add podophyllotoxin (0.29 mmol), 4-dimethylaminopyridine (0.46 mmol) and 4-thiazole formic acid (0.29 mmol), then add N,N-dimethylformamide (5 mL), stir at 25°C for 10 minutes, then add 1-(3-dimethylaminopropyl)-3-ethyl carbon in batches Diimine hydrochloride (0.58 mmol). Under the protection of nitrogen, the reaction solution was stirred and reacted at 25°C 4 Hour. The reaction solution was poured into water, stirred to precipitate a solid, filtered with suction, washed with water, and dried in vacuo to obtain a crude product. The crude product was purified by column chromatography to obtain a white solid product 4α-(thiazole-4-yl)-4-deoxy-podophyllotoxin (1), yield 72%.
[0027] Mp: 251~253℃; 1 H NMR (400 MHz, CDCl 3 ) δ 8.89 (d, J = 1.6 Hz, 1H), 8.27 (d, J = 1.6 Hz, 1H), 6.88 (s, 1H), 6.57 (s, 1H), 6.44 (s, 2H), 6.14 (d ,J = 8.4 Hz, 1H), 5.97 (s, 2H), 4.64 (d, J = 2.4 Hz, 1H)...
Embodiment 2
[0028] Example 2: Preparation of 4α-(1-methylpyrazole-4-yl)-4-deoxy-podophyllotoxin (2)
[0029] Add podophyllotoxin (0.29 mmol), 4-dimethylaminopyridine (0.46 mmol) and 1-Methyl Pyrazole-4-carboxylic acid (0.29 mmol), then add N,N-dimethylformamide (5 mL), stir at 25°C for 10 minutes, then add 1-(3-dimethylaminopropyl)-3-ethyl carbon in batches Diimine hydrochloride (0.58 mmol). Under the protection of nitrogen, the reaction solution was stirred and reacted at 25°C 6 Hour. The reaction solution was poured into water, stirred to precipitate a solid, filtered with suction, washed with water, and dried in vacuo to obtain a crude product. The crude product was purified by column chromatography to obtain a white solid product 4α-(1-methylpyrazole-4-yl)-4-deoxy-podophyllotoxin Su (2), yield 70%.
[0030] Mp: 126~128℃; 1 H NMR (400 MHz, CDCl 3) δ 7.88 (d, J = 11.6 Hz, 2H), 6.82 (s, 1H), 6.55 (s, 1H), 6.41 (s, 2H), 6.02 (d, J = 8.4 Hz, 1H), 5.97 (d ,J = 8.0 Hz, 2H), 4.6...
Embodiment 3
[0031] Example 3: Preparation of 4α-(1-methylimidazol-4-yl)-4-deoxy-podophyllotoxin (3)
[0032] Add podophyllotoxin (0.29 mmol), 4-dimethylaminopyridine (0.46 mmol) and 1-Methyl imidazole-4-carboxylic acid (0.32 mmol), Then N,N-dimethylformamide (5 mL) was added, Stir for 5 minutes at 30°C Then 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (0.58 mmol) was added in batches. Under nitrogen protection, Reaction solution in Stir the reaction at 30°C for 4 hours . The reaction solution was poured into water, stirred to precipitate a solid, filtered with suction, washed with water, and dried in vacuo to obtain a crude product. The crude product was purified by column chromatography to obtain a white solid product 4α-(1-Methylimidazol-4-yl)-4-deoxy-podophyllotoxin (3), yield 57%.
[0033] Mp: 143~145℃; 1 H NMR (400 MHz, CDCl 3 ) δ 7.58 (s, 1H), 7.50 (s, 1H),6.85 (s, 1H), 6.52 (s, 1H), 6.43 (s, 2H), 6.06 (d, J = 7.2 Hz, 1H), 5.94 (s,2H), 4.60 (s, 1H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com