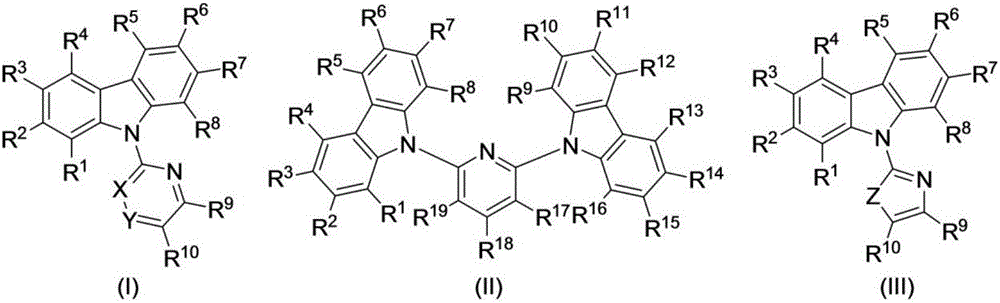
Preparation method of N-heteroaryl carbazole compounds
A technology for heteroaryl carbazoles and compounds is applied in the field of preparation of N-heteroarylcarbazole compounds, can solve the problems of requiring laboratory synthesis, high reaction temperature, long reaction time, etc., and achieves the reaction process and operation. Simple, broad application prospects, the effect of shortened response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
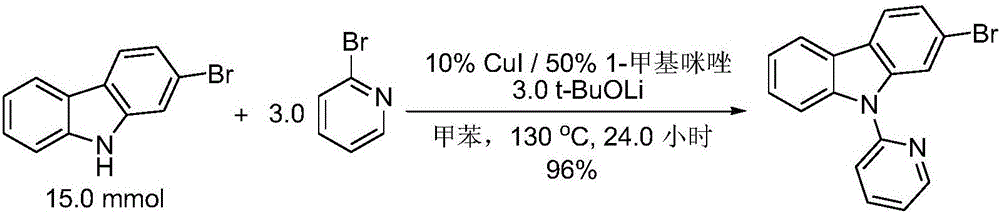
[0033] Embodiment 1: the preparation of 2-bromo-9-(2-pyridyl) carbazole (cuprous iodide is used as catalyst)
[0034]
[0035] Add 2-bromocarbazole (3.69g, 15.00mmol, 1.0eq), cuprous iodide (286mg, 1.5mmol, 0.1eq), and lithium tert-butoxide to a dry three-necked flask with a reflux condenser and a magnetic rotor. (3.60g, 45.00mmol, 3.0eq), pumped nitrogen three times, then added 2-bromopyridine (4.28mL, 45.00mmol, 3.0eq), 1-methylimidazole (595uL, 7.50mmol, 0.5eq) and toluene ( 56.6mL). The reaction mixture was refluxed at 130° C. for 24.0 hours, and monitored by TLC until the reaction of the raw material 2-bromocarbazole was completed. Quench with 30 mL of saturated sodium sulfite solution, filter, fully wash the insolubles with ethyl acetate, separate the organic phase in the mother liquor, extract the water phase with 30 mL of ethyl acetate three times, combine the organic phases, dry over anhydrous sodium sulfate, filter, and reduce pressure The solvent and excess 2-b...
Embodiment 2
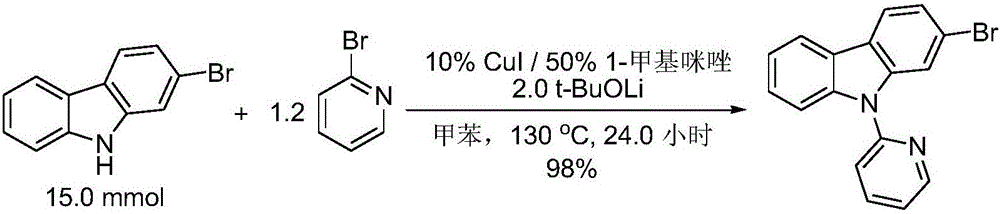
[0037] Embodiment 2: Preparation of 2-bromo-9-(2-pyridyl)carbazole (cuprous iodide is used as catalyst)
[0038]
[0039] Add 2-bromocarbazole (3.69g, 15.00mmol, 1.0eq), cuprous iodide (286mg, 1.5mmol, 0.1eq), and lithium tert-butoxide to a dry three-necked flask with a reflux condenser and a magnetic rotor. (2.40g, 30.00mmol, 2.0eq), pumped nitrogen three times, then added 2-bromopyridine (1.71mL, 18.00mmol, 1.2eq), 1-methylimidazole (595uL, 7.50mmol, 0.5eq) and toluene ( 56.6mL). The reaction mixture was refluxed at 130° C. for 24.0 hours, and monitored by TLC until the reaction of the raw material 2-bromocarbazole was completed. Quench with 30 mL of saturated sodium sulfite solution, filter, fully wash the insolubles with ethyl acetate, separate the organic phase in the mother liquor, extract the water phase with 30 mL of ethyl acetate three times, combine the organic phases, dry over anhydrous sodium sulfate, filter, and reduce pressure The solvent and excess 2-bromop...
Embodiment 3
[0041] Embodiment 3: the preparation of 2-bromo-9-(2-pyridyl) carbazole (cuprous iodide is made catalyst)
[0042]
[0043] Add 2-bromocarbazole (3.69g, 15.00mmol, 1.0eq), cuprous iodide (286mg, 1.5mmol, 0.1eq), and lithium tert-butoxide to a dry three-necked flask with a reflux condenser and a magnetic rotor. (2.40g, 30.00mmol, 2.0eq), nitrogen was replaced three times, and then 2-bromopyridine (1.71mL, 18.00mmol, 1.2eq), 1-methylimidazole (298uL, 3.75mmol, 0.25eq) and toluene ( 56.6mL). The reaction mixture was refluxed at 130° C. for 24.0 hours, and monitored by TLC until the reaction of the raw material 2-bromocarbazole was completed. Quench with 30 mL of saturated sodium sulfite solution, filter, fully wash the insolubles with ethyl acetate, separate the organic phase in the mother liquor, extract the water phase with 30 mL of ethyl acetate three times, combine the organic phases, dry over anhydrous sodium sulfate, filter, and reduce pressure The solvent and excess 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com