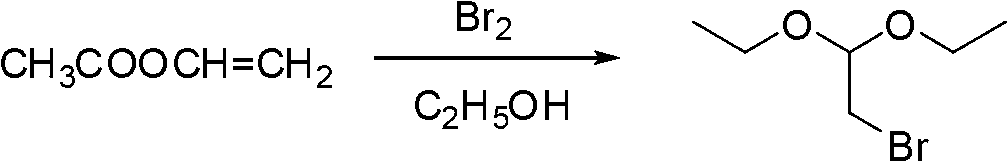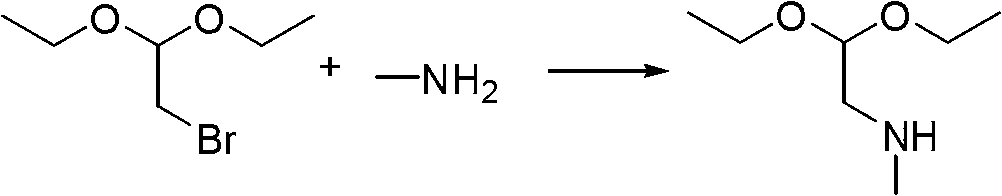Industrialization production method of 2-mercapto-1-methylimidazole
A technology of methylimidazole and its production method, which is applied in the field of industrialized production, can solve the problems of not finding a synthesis scheme of 2-mercapto-1-methylimidazole, and not giving a synthesis scheme of 2-mercapto-1-methylimidazole, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (a) Pump 480 kg of absolute ethanol and 180 kg of vinyl acetate into the No. 1 reactor. After the pumping is completed, nitrogen balances the vacuum in the reactor. During the reaction process, the temperature of the reaction solution was controlled at about 5°C, and 335 kg of bromine was pressed into the No. 1 reactor, and the addition was completed in about 10 hours. After the addition of bromine, the temperature was raised to 30°C, and the reaction was kept until the reaction liquid was sampled and GC detected that the intermediate was ≤ 1.0%, that is, the reaction was stopped.
[0020] Under stirring, transfer the pre-prepared 16.7% sodium carbonate aqueous solution into the No. 1 reaction kettle until the pH of the reaction solution is 8, then add 540 kg of dichloromethane, stir for 30 minutes, and separate the organic layer.
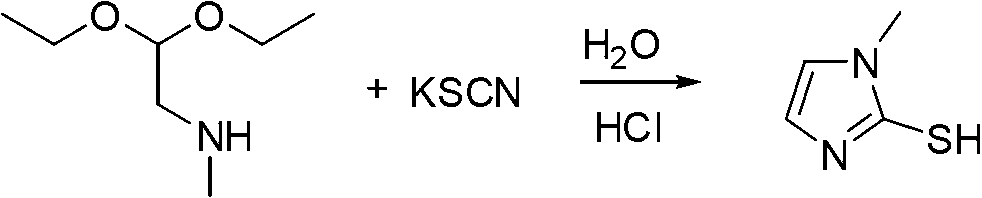
[0021] Add 300 kilograms of water and 60 kilograms of sodium chloride in No. 2 reactor, after stirring and dissolving completely, then add ...
Embodiment 2
[0027] (a) Pump 480 kg of absolute ethanol and 180 kg of vinyl acetate into the No. 1 reactor. After the pumping is completed, nitrogen balances the vacuum in the reactor. During the reaction process, the temperature of the reaction solution was controlled at about 5°C, and 335 kg of bromine was pressed into the No. 1 reactor, and the addition was completed in about 10 hours. After the addition of bromine, the temperature was raised to 30°C, and the reaction was kept until the reaction liquid was sampled and GC detected that the intermediate was ≤ 1.0%, that is, the reaction was stopped.
[0028] Under stirring, transfer the pre-prepared 16.7% sodium carbonate aqueous solution into the No. 1 reaction kettle until the pH of the reaction solution is 10, then add 540 kg of dichloromethane, stir for 30 minutes, and separate the organic layer.
[0029] Add 300 kilograms of water and 60 kilograms of sodium chloride in No. 2 reactor, after stirring and dissolving completely, then add...
Embodiment 3
[0035] (a) Pump 480 kg of absolute ethanol and 180 kg of vinyl acetate into the No. 1 reactor. After the pumping is completed, nitrogen balances the vacuum in the reactor. During the reaction process, the temperature of the reaction solution was controlled at about 5°C, and 335 kg of bromine was pressed into the No. 1 reactor, and the addition was completed in about 10 hours. After the addition of bromine, the temperature was raised to 30°C, and the reaction was kept until the reaction liquid was sampled and GC detected that the intermediate was ≤ 1.0%, that is, the reaction was stopped.
[0036] Under stirring, transfer the pre-prepared 16.7% sodium carbonate aqueous solution into the No. 1 reaction kettle until the pH of the reaction solution is 9, then add 540 kg of dichloromethane, stir for 30 minutes, and separate the organic layer.
[0037] Add 300 kilograms of water and 60 kilograms of sodium chloride in No. 2 reactor, after stirring and dissolving completely, then add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com