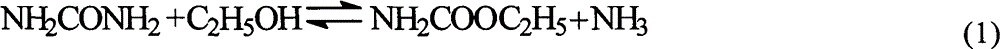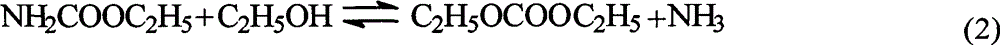Ion liquid catalyst for synthesizing diethyl carbonate by urea alcoholysis and preparation method thereof
A technology of diethyl carbonate and ionic liquid, which is applied in the field of ionic liquid catalyst and its preparation, can solve problems such as safety hazards and equipment corrosion, and achieve the effects of good stability, low price and high catalyst activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Catalyst preparation:
[0019] Under the protection of nitrogen, weigh 13.9736g of BMIMCl into a three-necked flask, add 10.9040g of zinc chloride and 7.6168g of magnesium chloride respectively, react at 105°C for 48h, and put it in a desiccator for later use.
[0020] Application experiment one:
[0021] Weigh 20.86g of urea, 200ml of absolute ethanol and 7.23g of catalyst into the autoclave, at 190°C, the stirring rate is 900r / min, react for 6h, take out the reaction product after cooling, analyze and calculate the DEC by gas phase internal standard method yield.
[0022] Based on urea, the yield of DEC is 22.08%.
[0023] Application experiment two:
[0024] Weigh 20.86g of urea, 200ml of absolute ethanol and 9.04g of catalyst into the autoclave, at 190°C, the stirring rate is 800r / min, react for 6h, take out the reaction product after cooling, analyze and calculate the DEC by gas phase internal standard method yield.
[0025] Based on urea, the yield of DEC is ...
Embodiment 2
[0030] Catalyst preparation:
[0031] Under nitrogen protection, weigh 8.7335g of 1-methyl-3-butylimidazole chloride into a three-necked flask, add 13.6295g of zinc chloride, react at 110°C for 48h, and put it in a desiccator for later use.
[0032] Application experiment one:
[0033] Weigh 20.86g of urea, 200ml of absolute ethanol and 9.04g of catalyst into the autoclave, at 210°C, the stirring rate is 900r / min, react for 5h, take out the reaction product after cooling, analyze and calculate the DEC by gas phase internal standard method yield.
[0034] Based on urea, the yield of DEC is 20.32%.
[0035] Application experiment two:
[0036] Weigh 20.86g of urea, 200ml of absolute ethanol and 9.04g of catalyst into the autoclave, raise the temperature to 230°C, stir at 900r / min, and react for 1h. Then the temperature was lowered to 200°C, the stirring rate was 800r / min, and the reaction was carried out for 4h. After cooling, the reaction product was taken out, and the yie...
Embodiment 3-6
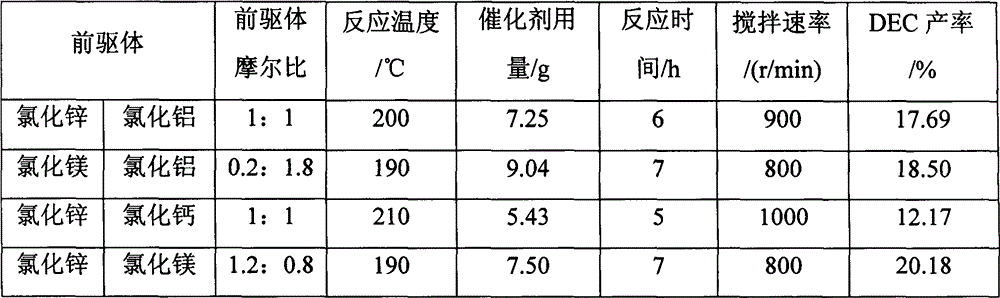
[0039] The selected precursors and reaction conditions are shown in Table 1, and the other reaction conditions are the same as those in Example 1 except that the added metal chlorides and their amounts are different. The catalysts prepared above were used to catalyze the alcoholysis of urea to synthesize DEC, and the yield of DEC was analyzed and calculated by the gas phase internal standard method, as shown in Table 1.
[0040] Table 1 Effects of different catalyst ratios and reaction conditions on the synthesis of DEC
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com