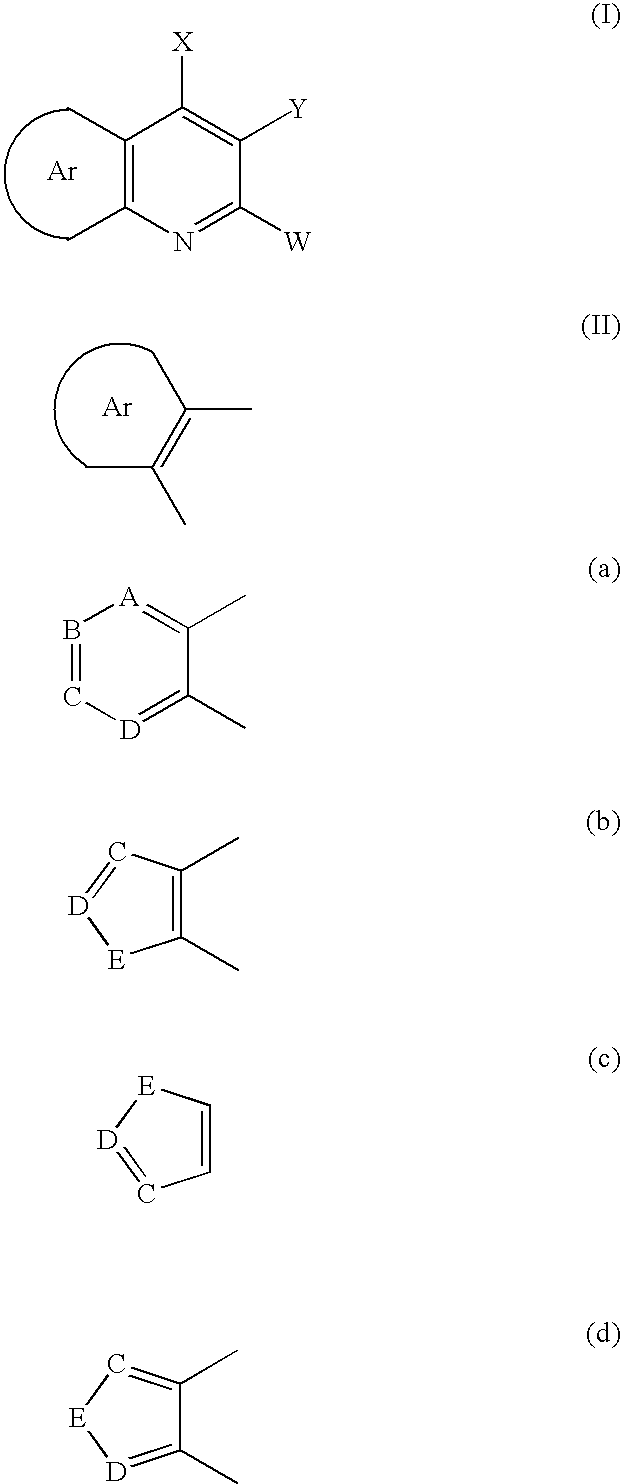
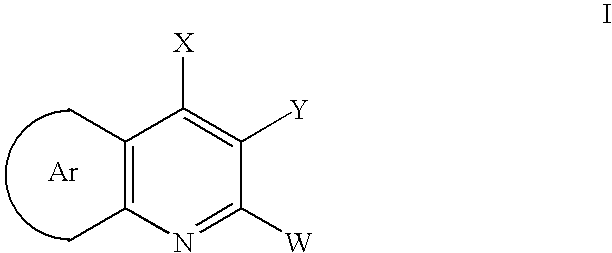
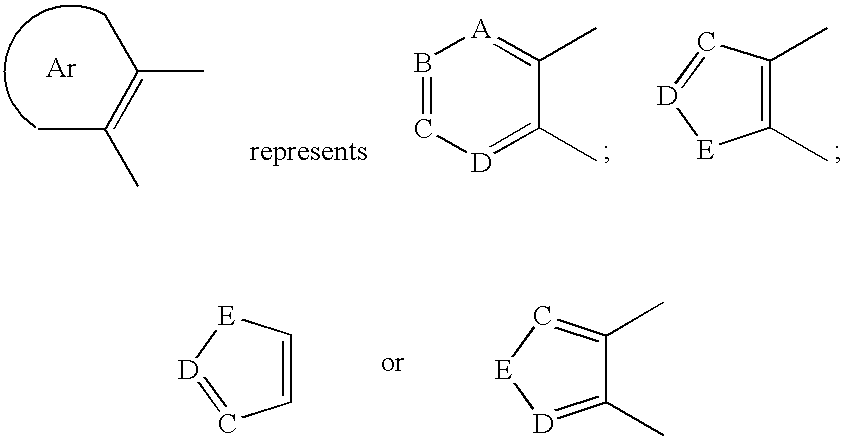
Aryl fused 2,4-disubstituted pyridines: NK3 receptor ligands
a technology of pyridine and aryl fused pyridine, which is applied in the field of aryl fused 2, 4disubstituted pyridines, can solve the problem of limiting the potential to evaluate these compounds in many appropriate disease models
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example i
[0118]
[0119] To a mixture of 20 g of 4-Aminopyridine and 37 mL of triethylamine in 380 mL of methylene chloride is added 28.8 mL of pivaloyl chloride in a dropwise fashion. After 1 hour at room temperature the reaction mixture is washed with water and sodium bicarbonate solution, dried over magnesium sulfate and the solvent is removed in vacuo. The residue is triturated with hexane to afford 4-trimethylacetamidopyridine as a white solid.
example ii
[0120]
[0121] A solution of 10 g of 4-trimethylacetamido pyridine in 150 mL of dry tetrahydrofuran is cooled to −78° C. and 88 mL of 1.6M n-butyllithium in hexane is added in a dropwise fashion. The mixture is then stirred at 0° C. for 3 hours, cooled again to −78° C. and 21.3 g of diethyl oxalate dissolved in 190 mL of tetrahydrofuran is added in a dropwise fashion. the reaction mixture was then gradually allowed to come to room temperature. The reaction mixture is diluted with water and the product is extracted with ether. After drying over magnesium sulfate the solvent is removed in vacuo. The residue is chromatagraphed on silica gel with hexane / ether 2:1 as the eluent to afford ethyl 4-trimethylacetamido-3-pyridylpyruvate as an orange oil.
example iii
[0122]
[0123] A mixture of 3.59 g of ethyl 4-trimethylacetamido-3-pyridylpyruvate, 2.89 g of potassium hydroxide in 10 mL of ethanol and 40 mL of water is heated at reflux for 2 hours. At this time 3.1 g of acetophenone is added and refluxing is continued for 6 hours. The ethanol is removed in vacuo, the resulting aqueous solution is washed with ether and the aqueous layer is acidified to pH5. The product is collected washed with water and dried to afford (2-phenylpyridino[4,3-b]pyridin-4-yl)carboxylic acid as a white solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com