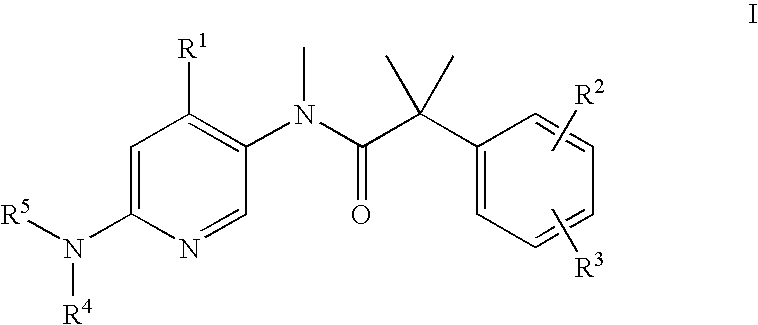
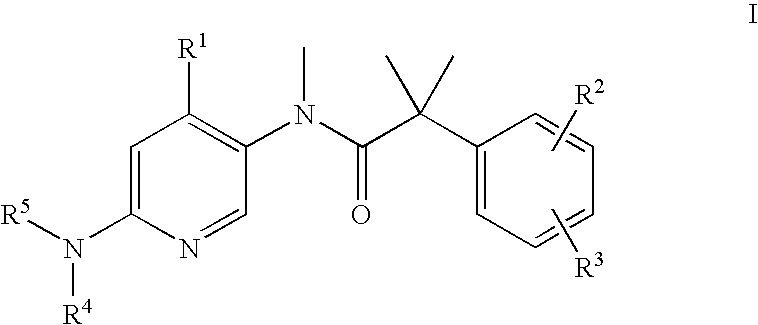
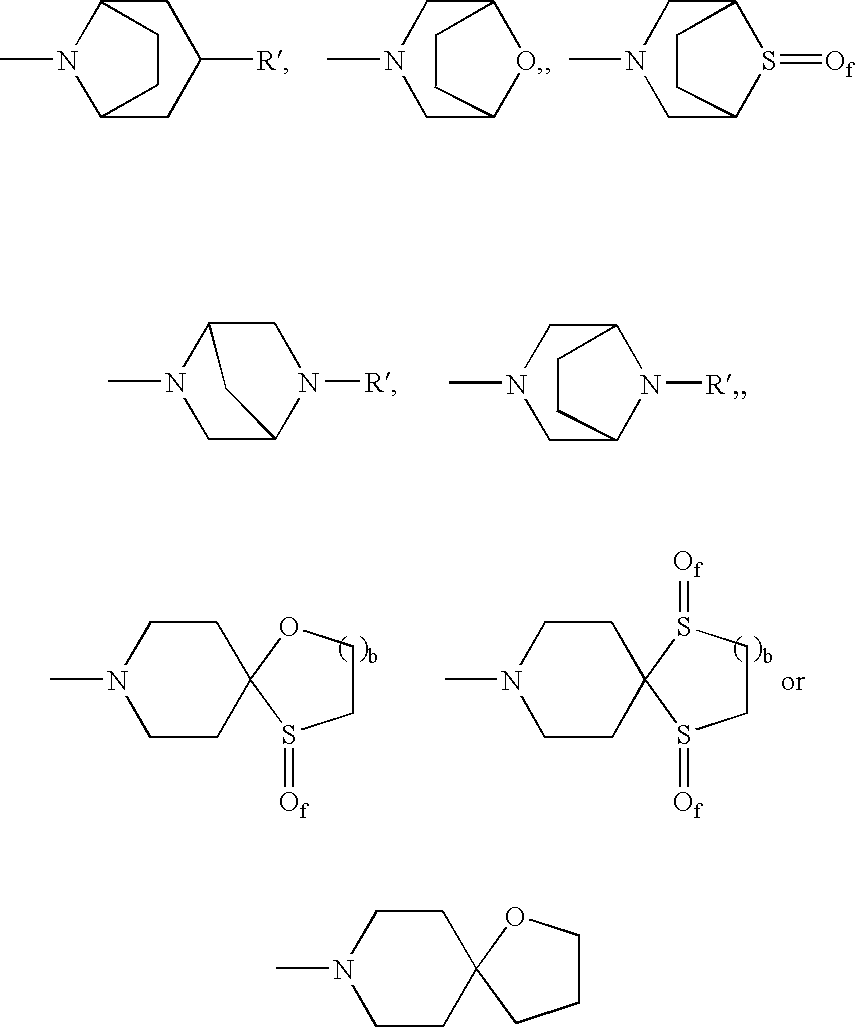
Dual NK1/NK3 receptor antagonists
a technology of nk1/nk3 receptor and antagonist, which is applied in the field of neuropsychiatric disorders, can solve the problems of hampered efforts and lack of knowledge about the cause and nature of this diseas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N-[4-(2-Chloro-phenyl)-6-(2-hydroxy-ethylamino)-pyridin-3-yl]-2-(3,5-dichloro-phenyl)-N-methyl-isobutyramide
a) N-[6-Chloro-4-(2-chloro-phenyl)-pyridin-3-yl]-2-(3,5-dichloro-phenyl)-N-methyl-isobutyramide
[1445] To a solution of 1.20 g (4.74 mmol) [6-chloro-4-(2-chloro-phenyl)-pyridin-3-yl]-methyl-amine and 1.43 g (5.68 mmol) 2-(3,5-dichloro-phenyl)-2-methyl-propionyl chloride in 20 ml toluene 1.27 g (10.42 mmol) 4-dimethylaminopyridine was added and the resulting solution stirred at 120° for 48 h. After cooling to ambient temperature, the solution was poured into 100 ml 0.5 N NaHCO3-solution and extracted three times with 50 ml CH2Cl2. The combined organic layers were dried (Na2SO4), filtered and evaporated. The residue was purified by flash-chromatography (SiO2, hexanes / ethyl acetate 4:1) to give 1.90 g (85%) of the title compound as a white solid.
[1446] MS m / e (%): 469.1 (M+H+, 100).
b) N-[4-(2-Chloro-phenyl)-6-(2-hydroxy-ethylamino)-pyridin-3-yl]-2-(3,5-dichloro-phenyl)-N-met...
example 2
(S)-N-[4-(2-Chloro-phenyl)-6-(2-hydroxymethyl-pyrrolidin-1-yl)-pyridin-3-yl]-2-(3,5-dichloro-phenyl)-N-methyl-isobutyramide
[1449] To a solution of 0.15 g (0.32 mmol) N-[6-chloro-4-(2-chloro-phenyl)-pyridin-3-yl]-2-(3,5-dichloro-phenyl)-N-methyl-isobutyramide and 0.25 g (2.47 mmol) L-prolinol in 2 ml dimethyl sulfoxide 0.2 g (1.55 mmol) Na2CO3 was added and the solution was stirred at 130° C. for 22 h. After cooling to ambient temperature, the solution was poured into 20 ml 0.5 N NaHCO3-solution and extracted three times with 30 ml CH2Cl2. The combined organic layers were dried (Na2SO4), filtered and evaporated. The residue was purified by flash-chromatography (SiO2, CH2Cl2 / ethyl acetate 1:2) to give 0.15 g (87%) of the title compound as a white foam.
[1450] MS m / e (%): 532.2 (M+H+, 100).
example 3
(2S,4R)-N-[4-(2-Chloro-phenyl)-6-(4-hydroxy-2-hydroxymethyl-pyrrolidin-1-yl)-pyridin-3-yl]-2-(3,5-dichloro-phenyl)-N-methyl-isobutyramide
[1451] To a solution of 0.15 g (0.32 mmol) N-[6-chloro-4-(2-chloro-phenyl)-pyridin-3-yl]-2-(3,5-dichloro-phenyl)-N-methyl-isobutyramide and 0.25 g (2.13 mmol) (2S,4R)-4-hydroxy-2-hydroxymethyl-pyrrolidine in 2 ml dimethyl sulfoxide 0.2 g (1.55 mmol) Na2CO3 was added and the solution was stirred at 130° C. for 9 h After cooling to ambient temperature, the solution was poured into 20 ml 0.5 N NaHCO3-solution and extracted three times with 30 ml CH2Cl2. The combined organic layers were dried (Na2SO4), filtered and evaporated. The residue was purified by flash-chromatography (SiO2, CH2Cl2 / ethyl acetate 1:2) to give 0.05 g (31%) of the title compound as a white foam.
[1452] MS m / e (%): 548.3 (M+H+, 100).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com