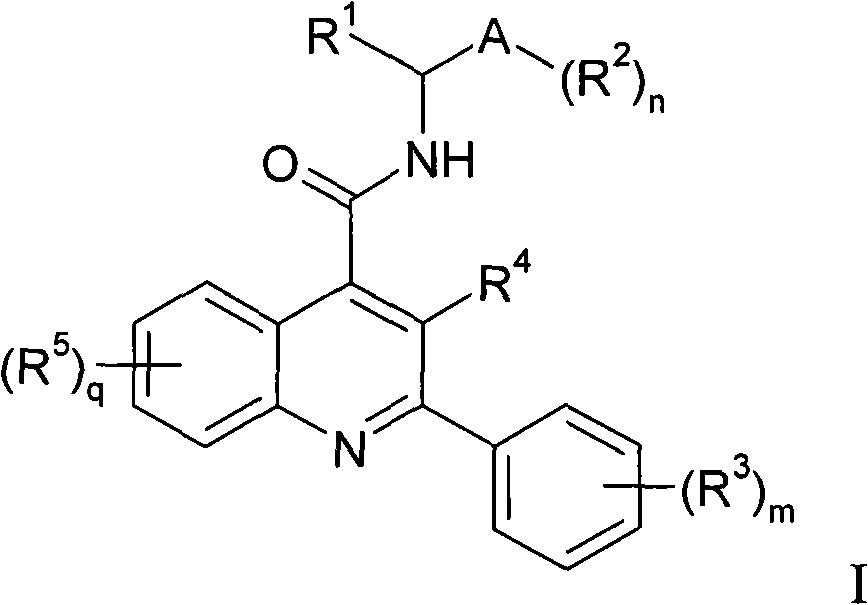
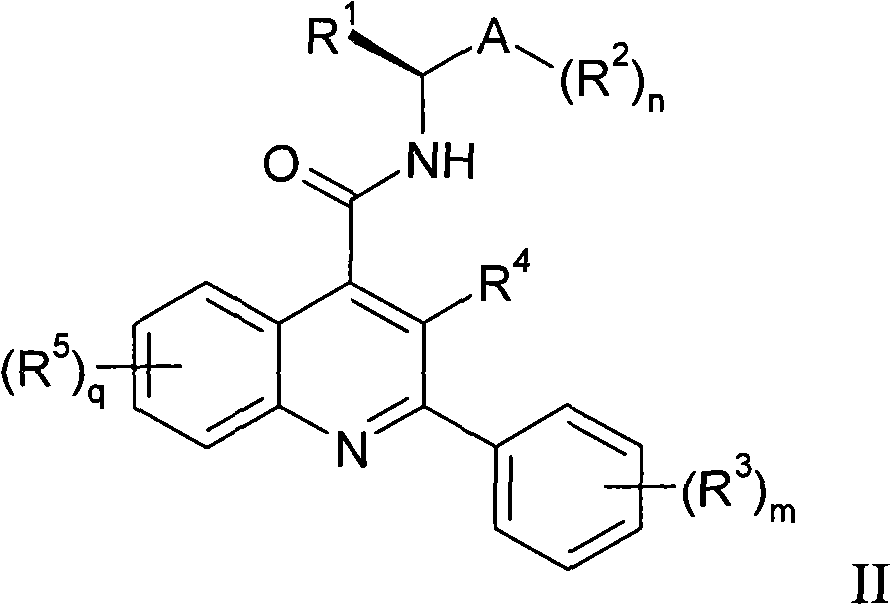
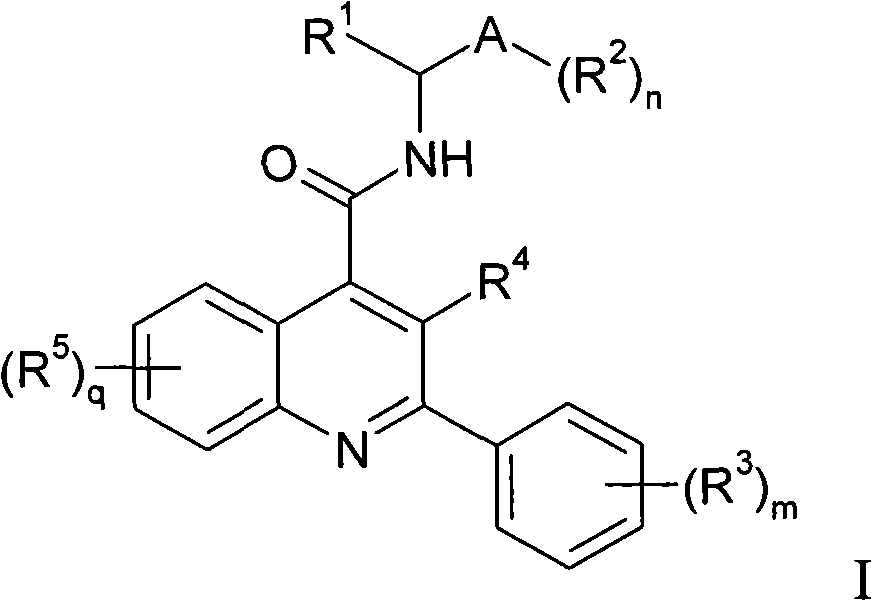
Alkylpyridyl quinolines as NK3 receptor modulators
A technology of alkyl and pyridine, applied in the field of alkyl pyridyl quinoline derivatives, which can solve problems such as limited evaluation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 37
[0165] Example 37: 2-Phenyl-N-[(1S)-1-phenylpropyl]-3-(pyridin-4-ylmethyl)quinoline-4-formyl Amines (1)
[0166]
[0167] The compound of Example 37 was prepared according to the following scheme:
[0168]
[0169] (a) N-methoxy-N-methyl-3-pyridin-4-yl-propionamide
[0170]
[0171] To a mixture of 3-pyridin-4-ylpropionic acid (3.91 g, 25.8 mmol) and N-hydroxybenzotriazole (3.49 g, 25.8 mmol) in N,N-dimethylformamide (75 mL), Add N,N'-dicyclohexylcarbodiimide (5.33 g, 25.8 mmol). After stirring the mixture for 4 h, N,O-dimethylhydroxylamine hydrochloride (3.78 g, 38.8 mmol) and triethylamine (9 mL, 64.6 mmol) were added, and the resulting slurry was shaken using a wrist shaker. Mix overnight. The mixture was quenched by the addition of water (10 mL) and concentrated under reduced pressure with heating at 70 °C to remove most of the N,N-dimethylformamide. The resulting slurry was partitioned between dichloromethane and water which had been acidified with 1M HCl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com