Synthetic peptide NK3R-A2 based on NK3 receptor and application thereof
A technology of NK3R-A2, synthetic peptide, applied in the field of tumor treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The synthetic peptide NK3R-A2 provided by the present invention has a molecular weight of 1536.70Da; the specific sequence is Cys-Asn-Gly-Arg-Cys-Gly-Gly-Asp-Phe-Phe-MePhe-Gly-Leu-Met-NH 2 ; or abbreviated as: CNGRC-GG-DFF(MeF)GLM-NH 2 . The synthetic peptide is prepared by the Fmoc solid-phase peptide synthesis method; taking a synthetic peptide NK3R-A2 of 0.2495 g in a certain synthesis as an example, the specific preparation steps are described in detail as follows.
[0048] (1) Weigh 0.3g Rink resin into the peptide synthesizer rinsed with DMF, then add 4mL DMF, let it stand for 30 minutes to make the resin fully swell, and then use a vacuum pump to remove the DMF; deprotection twice:
[0049] The deprotection refers to adding 4mL of deprotection solution (the volume ratio of piperidine to DMF is 1:3) into the synthesizer, stirring and reacting at 25°C~28°C for 20min, and vacuum pumping to dry;
[0050] (2) Wash the deprotected resin in step (1) in the following o...
Embodiment 2
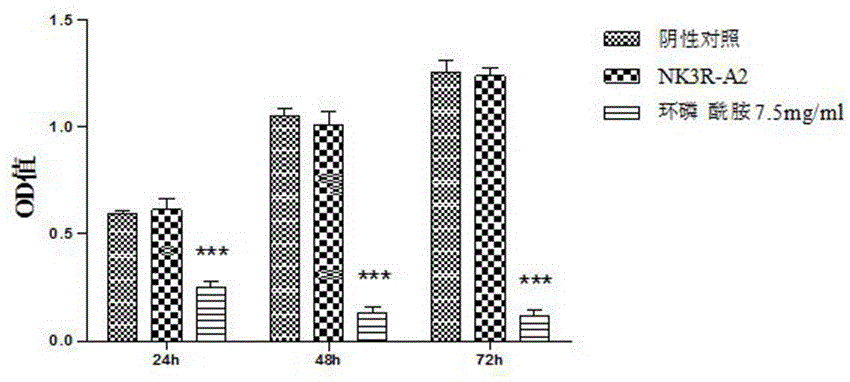
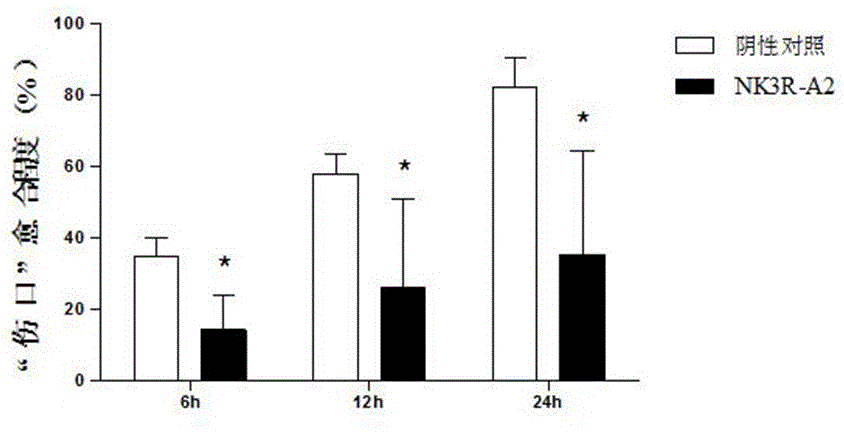
[0073] For the synthetic peptide NK3R-A2 prepared in Example 1, the inventors conducted specific experiments to verify its anti-tumor angiogenesis effect, and the relevant experiments are briefly introduced as follows.
[0074] one, Validation of anti-angiogenic effect in vitro
[0075] 1 , Cell proliferation assay
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com