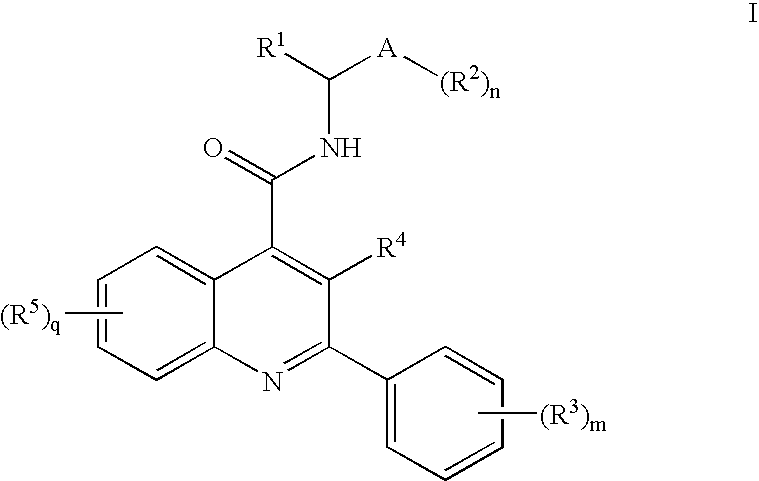
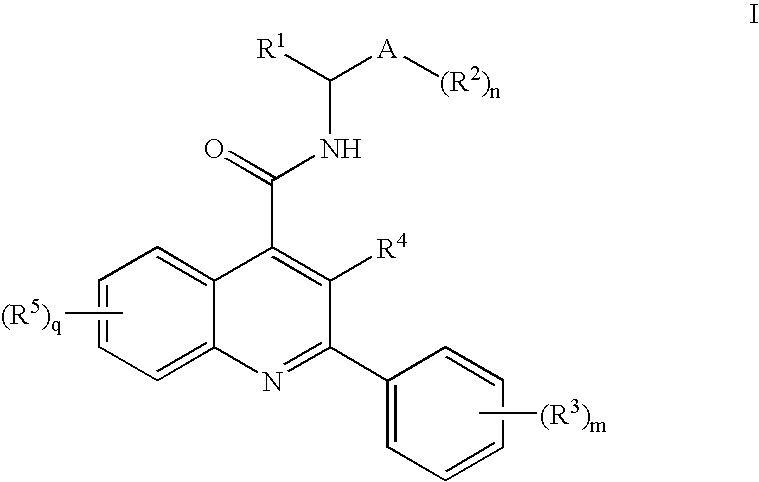
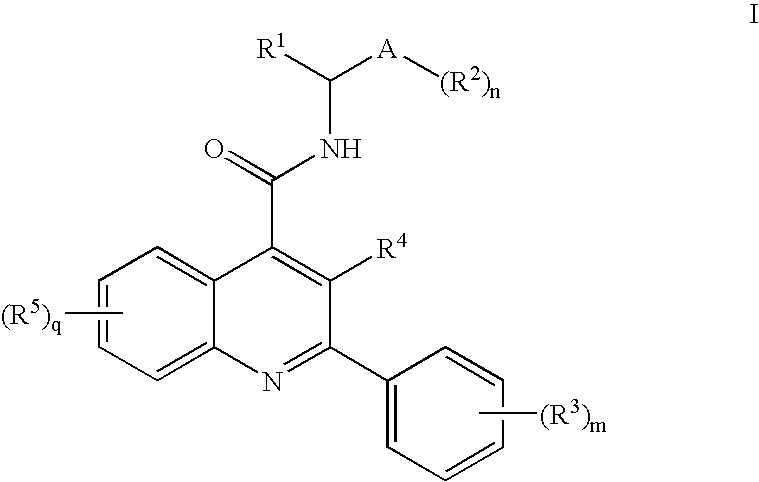
Alkylpyridyl Quinolines as Nk3 Receptor Modulators
a technology of nk3 receptor and alkylpyridyl quinoline, which is applied in the field of alkylpyridyl quinoline derivatives, can solve the problem of limiting the potential to evaluate these compounds in many appropriate disease models
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 37
2-Phenyl-N-[(1S)-1-phenylpropyl]-3-(pyridin-4-ylmethyl)quinoline-4-carboxamide (1)
[0146]
[0147]The compound of Example 37 was prepared in accord with the following scheme:
(a) N-Methoxy-N-methyl-3-pyridin-4-yl-propionamide
[0148]
[0149]To a mixture of 3-pyridin-4-ylpropionic acid (3.91 g, 25.8 mmol) and N-hydroxybenzotriazole (3.49 g, 25.8 mmol) in N,N-dimethylformamide (75 mL) was added N,N′-dicyclohexylcarbodiimide (5.33 g, 25.8 mmol). After agitating the mixture for 4 h, N,O-dimethylhydroxylamine hydrochloride (3.78 g, 38.8 mmol) and triethylamine (9 mL, 64.6 mmol) were added and the resulting slurry mixed overnight using a wrist shaker. The mixture was quenched by addition of water (10 mL) and concentrated under reduced pressure with heating at 70° C. to remove most of the N,N-dimethylformamide. The resulting slurry was partitioned between methylene chloride and water, which had been acidified with 1 M HCl. The aqueous layer was basified by addition of 1 M NaOH and extracted with me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com