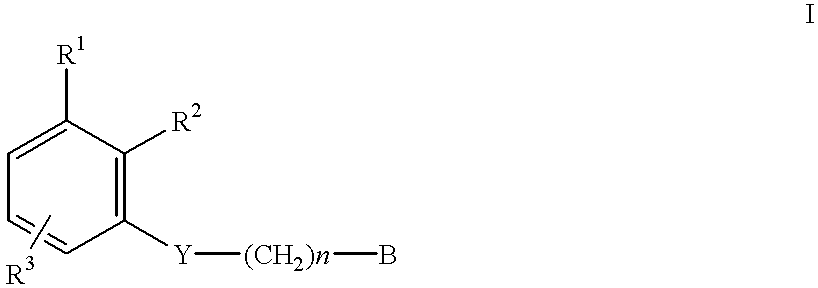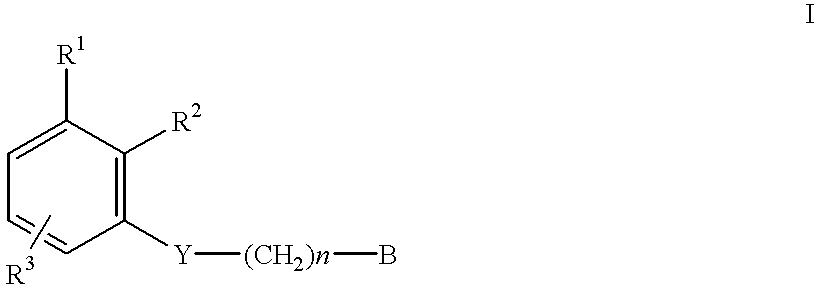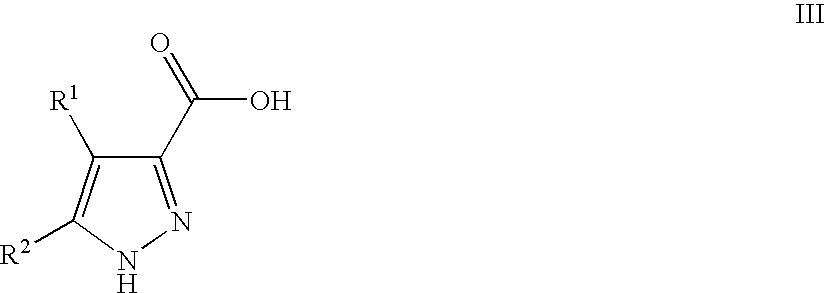Patents
Literature
121results about How to "Longer acting" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
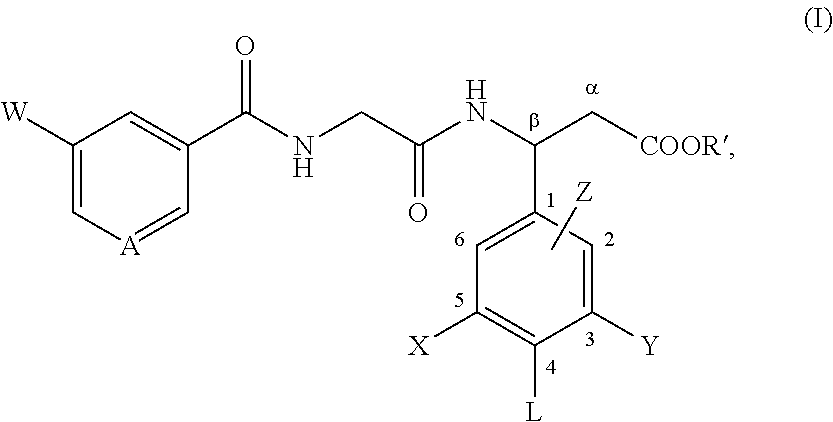
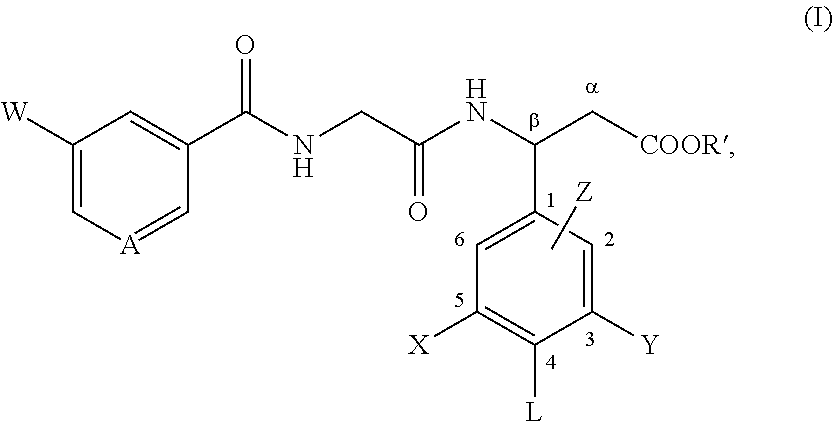
Use of angiotensin ii agonists
InactiveUS20120035232A1High expressionPromoting reinnervationOrganic active ingredientsBiocideMedicineAgonist
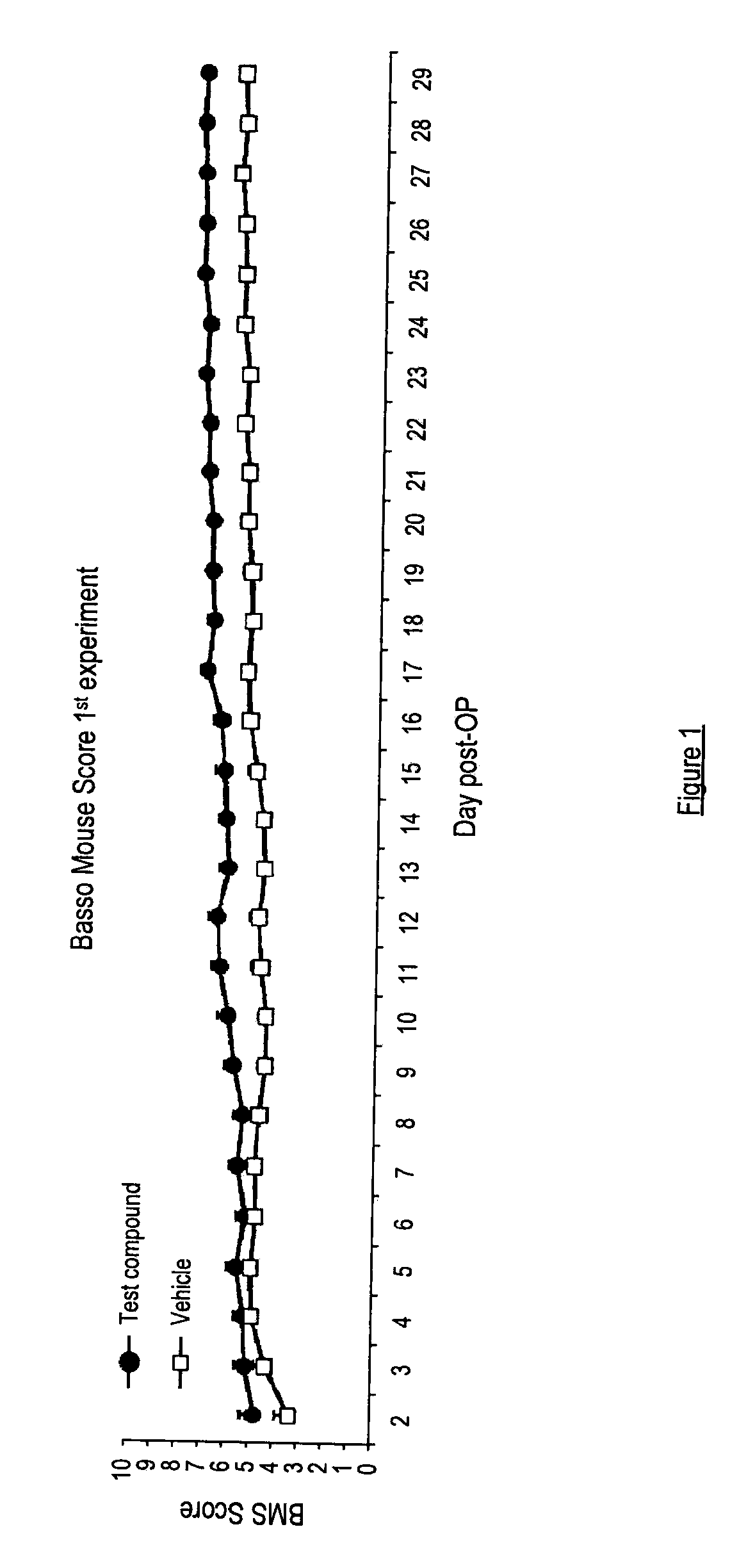
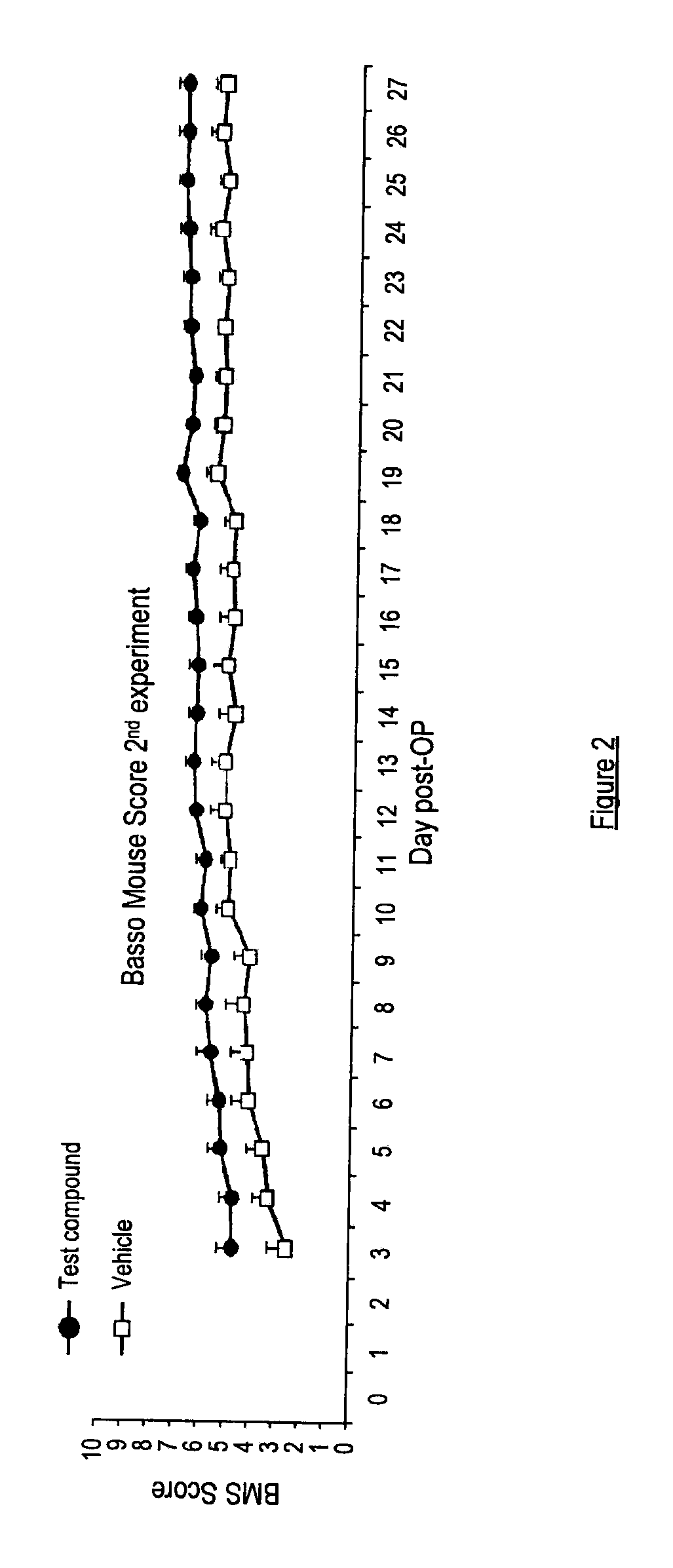
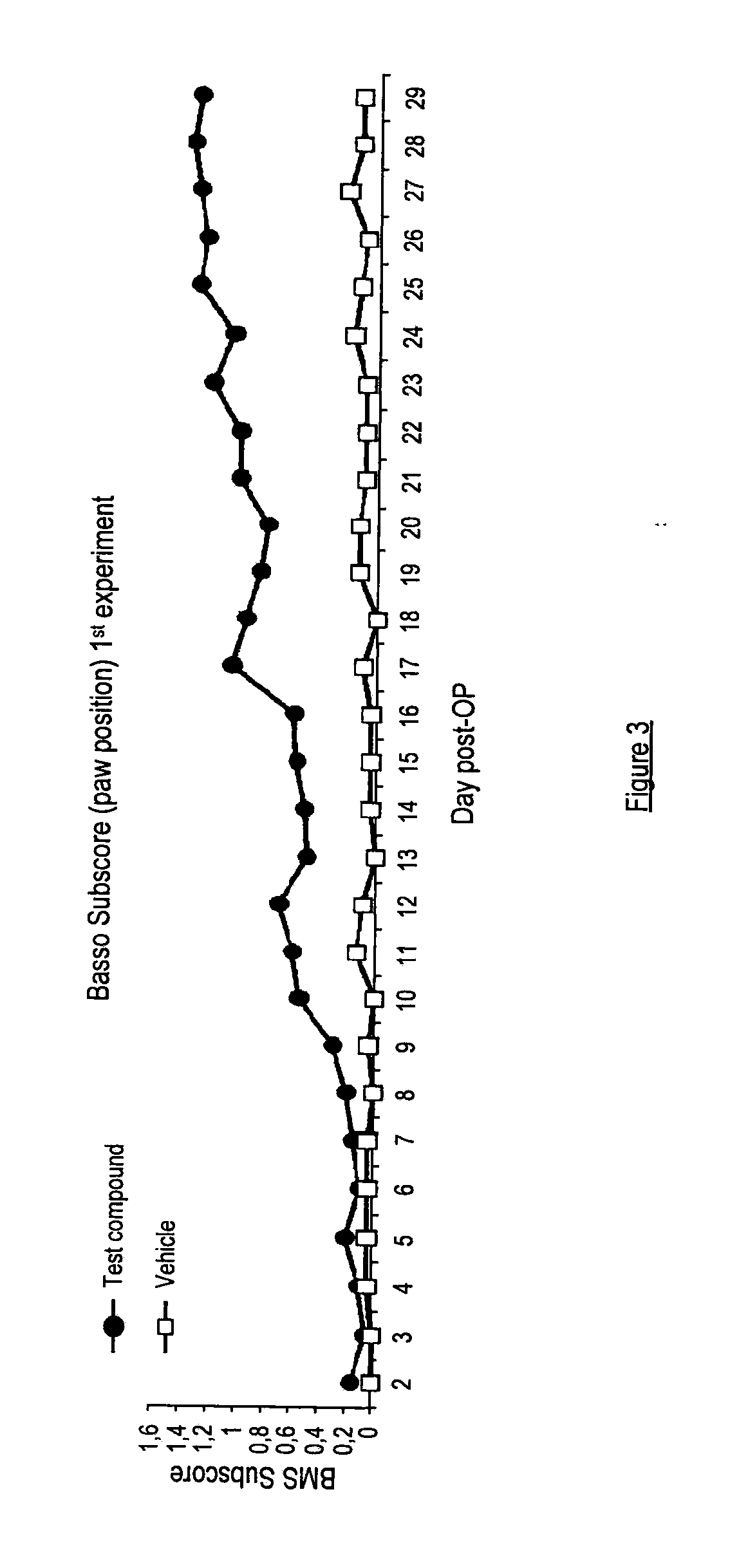
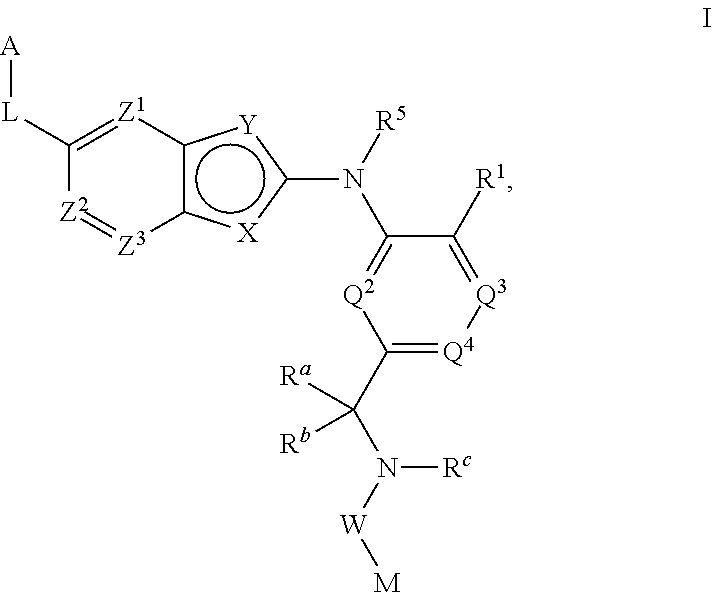
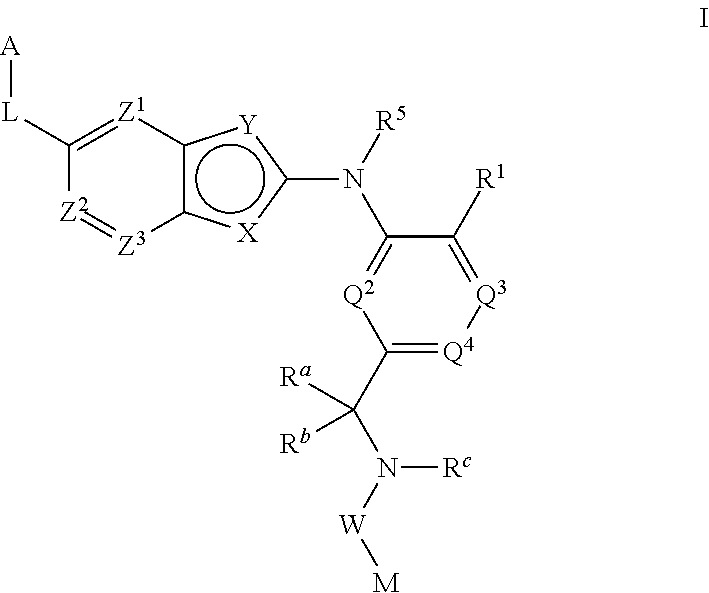
There is provided a compound of formula I,wherein Y1, Y2, Y3, Y4, Z1, Z2, R1, R2, R3, R4 and R5 have meanings given in the description, and pharmaceutically-acceptable salts thereof, for use in the treatment of spinal cord injury.
Owner:VICORE PHARMA AB
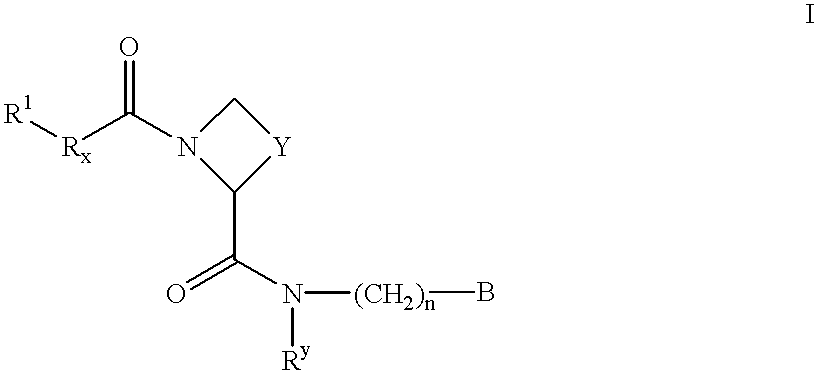
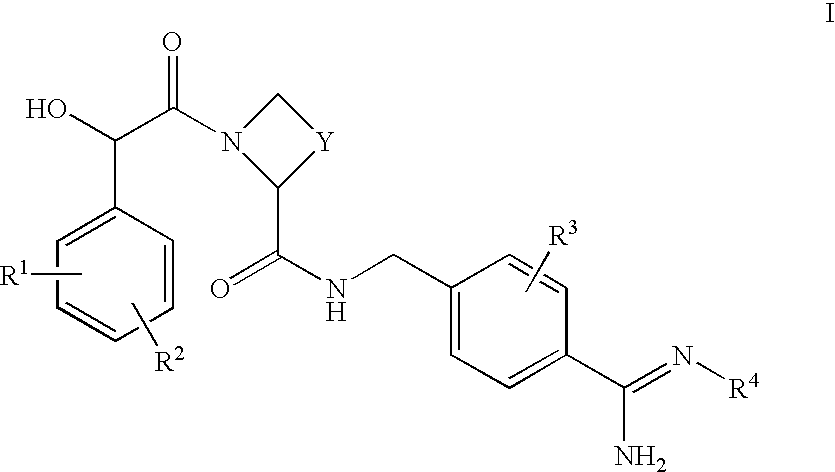
Amidino derivatives and their use as thrombin inhibitors
InactiveUS6221898B1More efficaciousToxic reductionBiocidePeptide/protein ingredientsAmidino derivativeThrombin activity
There is provided compounds of formula I,wherein R1, R2, R3, Y, n and B have meanings given in the description which are useful as competitive inhibitors of trypsin-like proteases, such as thrombin, and in particular in the treatment of conditions where inhibition of thrombin is required (e.g. thrombosis) or as anticoagulants.
Owner:ASTRAZENECA AB
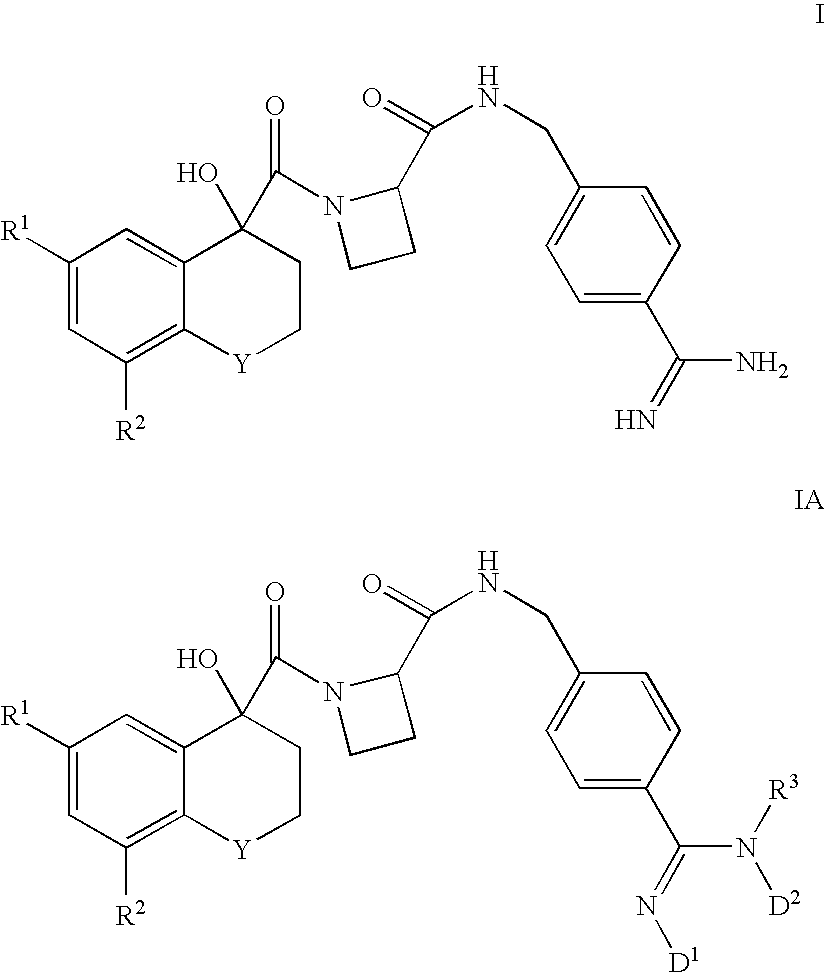
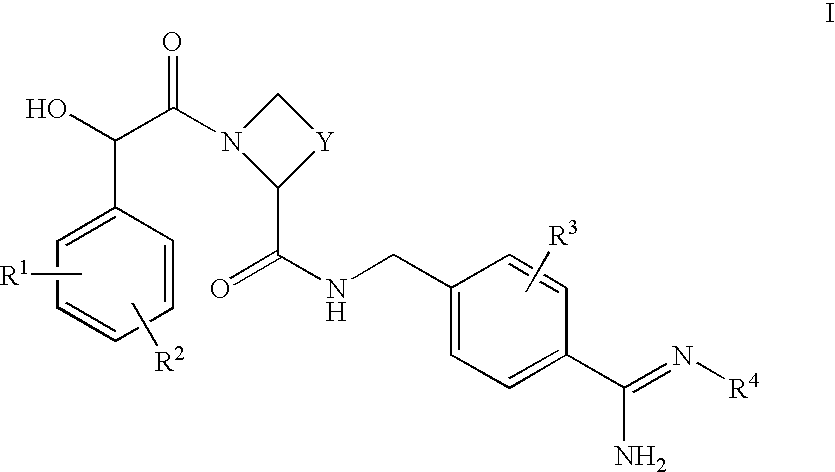
Thiochromane derivatives and their use as thrombin inhibitors
InactiveUS6716834B2Toxic reductionLonger actingOrganic active ingredientsBiocideThrombusPharmaceutical drug
There is provided compounds of formulae I and IAwherein Y, R<1>, R<2>, R<3>, D<1 >and D<2 >have meanings given in the description which are useful as, or as prodrugs of, competitive inhibitors of trypsin-like proteases, such as thrombin, and in particular in the treatment of conditions where inhibition of thrombin is required (e.g. thrombosis) or as anticoagulants.
Owner:ASTRAZENECA AB
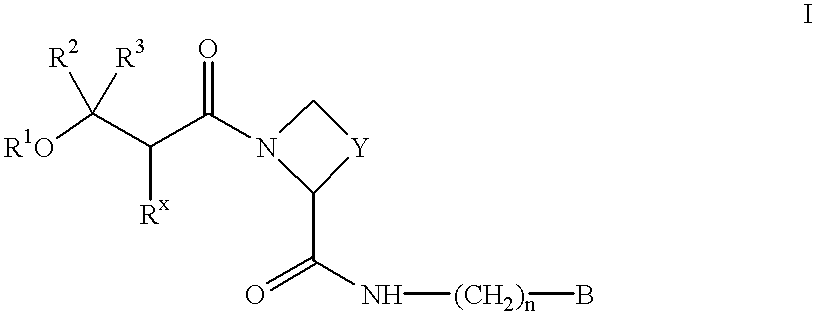
Amidino derivatives and their use as thrombin inhibitors
InactiveUS6265397B1More efficaciousToxic reductionBiocideOrganic chemistryThrombusAmidino derivative
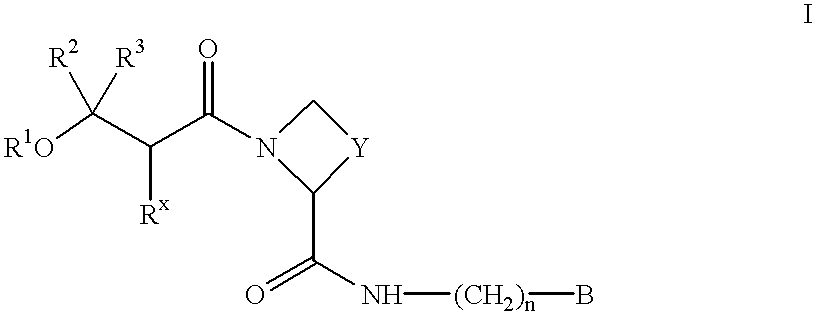
There is provided compounds of formula I,wherein R1, Rx, Y, Ry, n and B have meanings given in the description which are useful as competitive inhibitors of trypsin-like proteases, such as thrombin, and in particular in the treatment of conditions where inhibition of thrombin is required (e.g. thrombosis) or as anticoagulants.
Owner:ASTRAZENECA AB
INHIBITOR OF p38 MAP KINASE
ActiveUS20150232450A1Toxic reductionLonger actingPowder deliveryOrganic active ingredientsMedicineKinase
A compound has the following formula:The compound is a p38 MAP kinase inhibitor. The compound and its pharmaceutically acceptable salts can be used for treatment of conditions, such as inflammatory diseases.
Owner:RESPIVERT
Abuse resistant formula
ActiveUS20120015007A1Avoidance and reduction of riskGood sustained release effectBiocideNervous disorderChronic painFilm-forming agent
There is provided a sustained-release pharmaceutical composition comprising a solid, continuous network comprising an excipient with a high mechanical strength, which network also comprises pores, within which pores is interspersed a mixture of an active ingredient and a film-forming agent, characterised in that said pores are formed during the production of the composition. Compositions of the invention find particularly utility as abuse-resistant formulations comprising opioid analgesics that may be employed in the treatment of chronic pain.
Owner:EMPLICURE AB
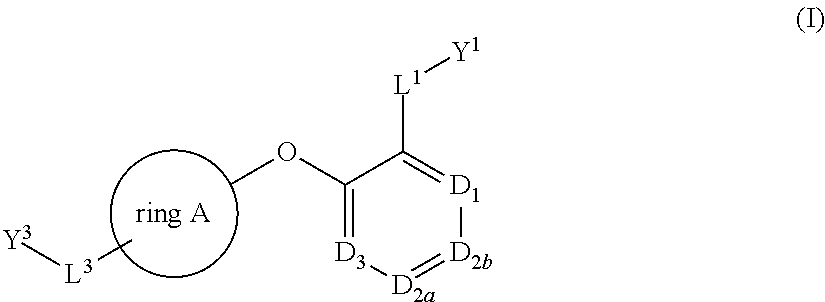
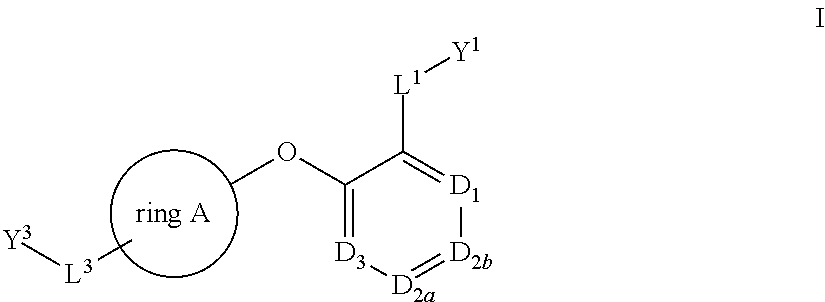
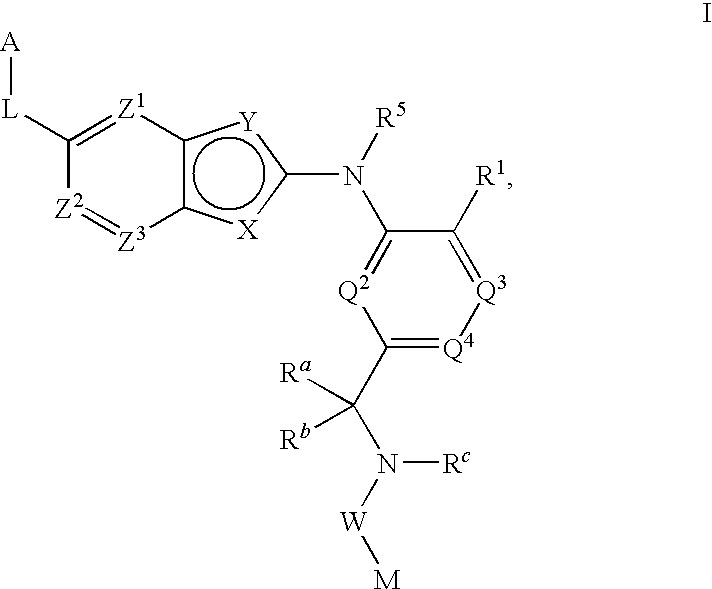
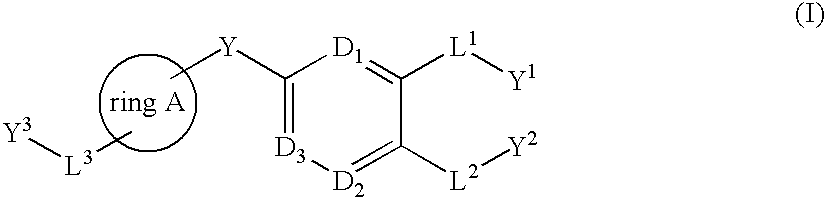
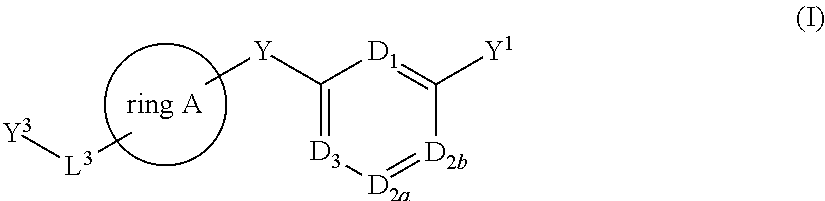
Bis-aryl compounds for use as medicaments
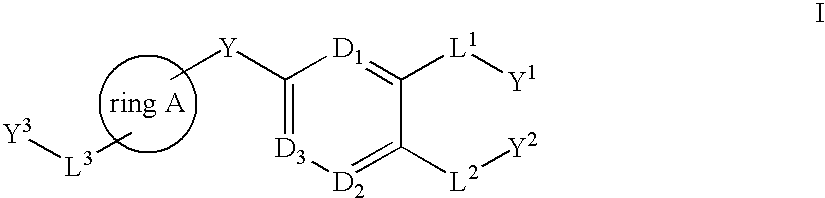
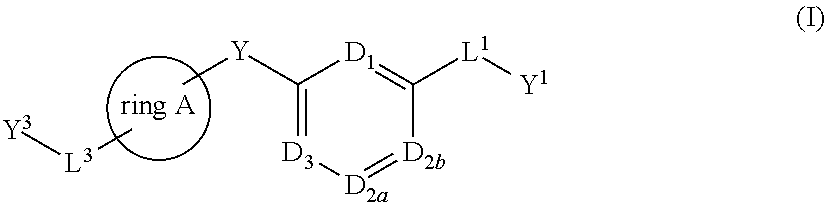
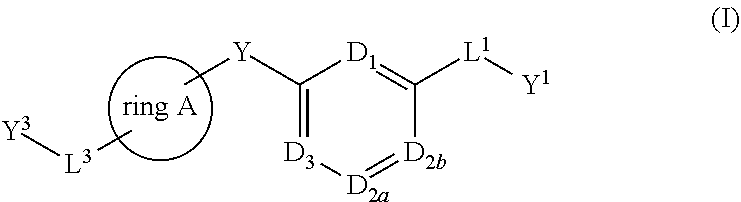
There is provided compounds of formula (I), wherein ring A, D1, D2a, D2b, D3, L1, Y1, L3 and Y3 have meanings given in the description, and pharmaceutically-acceptable salts thereof, which compounds are useful in the treatment of diseases in which inhibition of leukotriene C4 synthase is desired and / or required, and particularly in the treatment of a respiratory disorder and / or inflammation.
Owner:BIOLIPOX AB
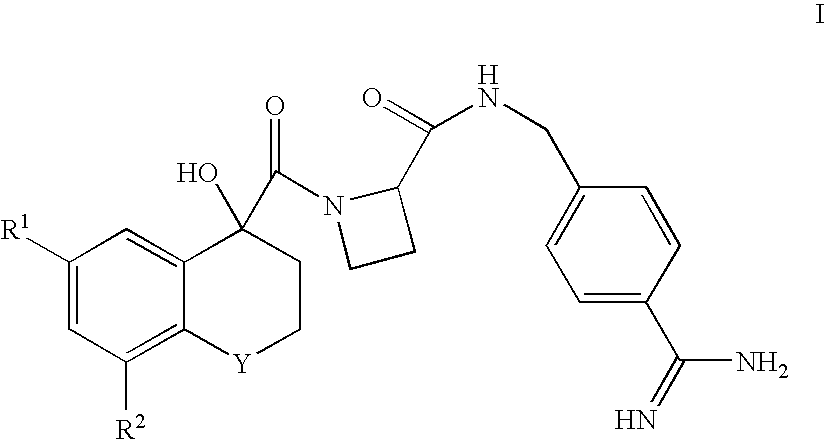
Amidinobenzylamine derivatives and their use as thrombin inhibitors
InactiveUS6599894B1Longer actingWide range of activitiesBiocidePeptide/protein ingredientsAnticoagulantThrombin activity
There is provided compounds of formula Iwherein R1, R2, Y, R3 and R4 have meanings given in the description which are useful as, or as prodrugs of, competitive inhibitors of trypsin-like proteases, such as thrombin, and in particular in the treatment of conditions where inhibition of thrombin is required (e.g. thrombosis) or as anticoagulants.
Owner:ASTRAZENECA AB
Amino acid derivatives and their use as thrombin inhibitors
InactiveUS6255301B1More efficaciousToxic reductionSilicon organic compoundsBiocideAnticoagulantThrombin activity
There is provided compounds of formula I,wherein R1, R2, R3, Rx, Y, n and B have meanings given in the description which are useful as competitive inhibitors of trypsin-like proteases, such as thrombin, and in particular in the treatment of conditions where inhibition of thrombin is required as in thrombosis or as anticoagulants.
Owner:ASTRAZENECA AB
Positive modulators of nicotinic acetylcholine receptors
InactiveUS20070179172A1More efficaciousLonger actingBiocideNervous disorderCompound (substance)Stereochemistry
Owner:ASTRAZENECA AB
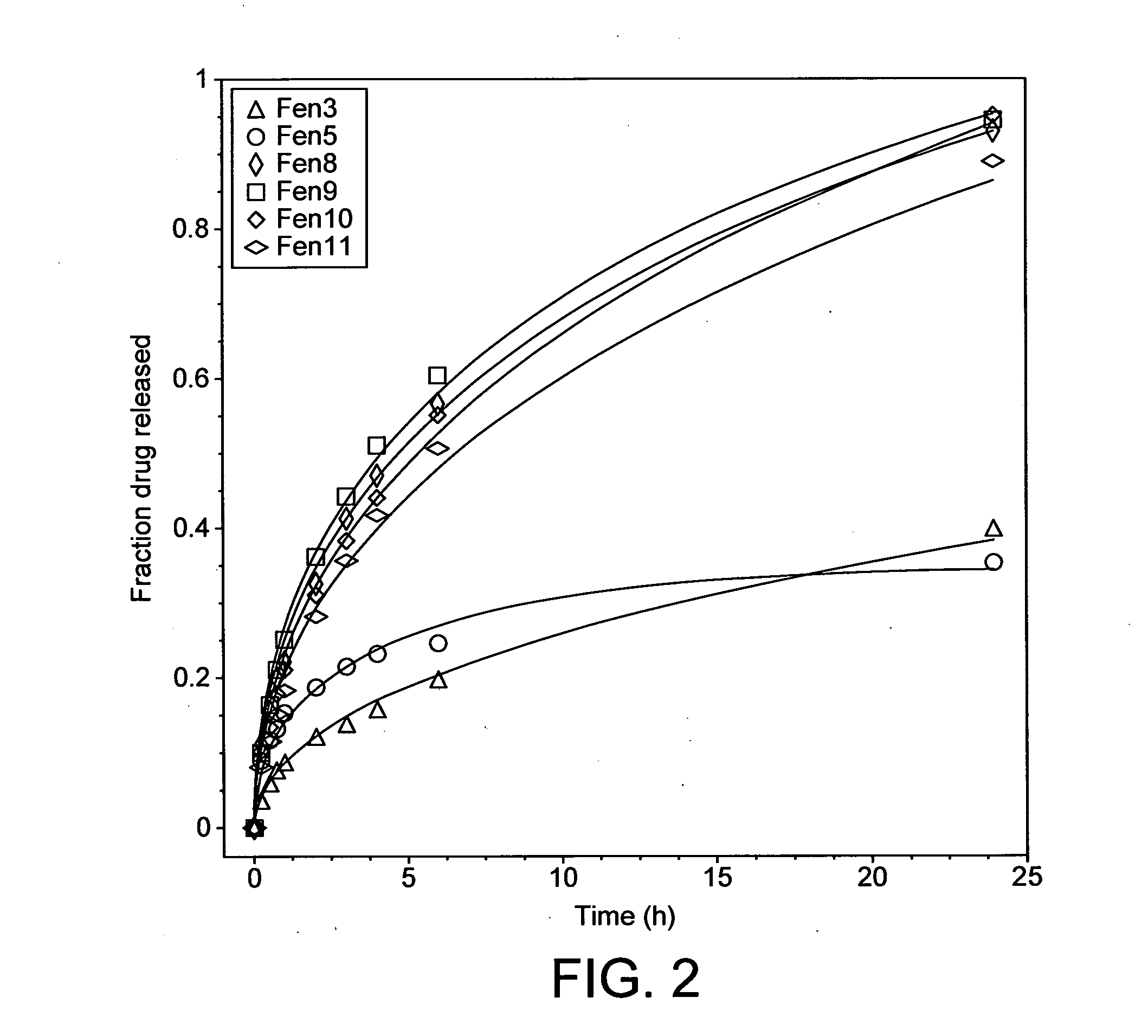
Breakthrough pain management
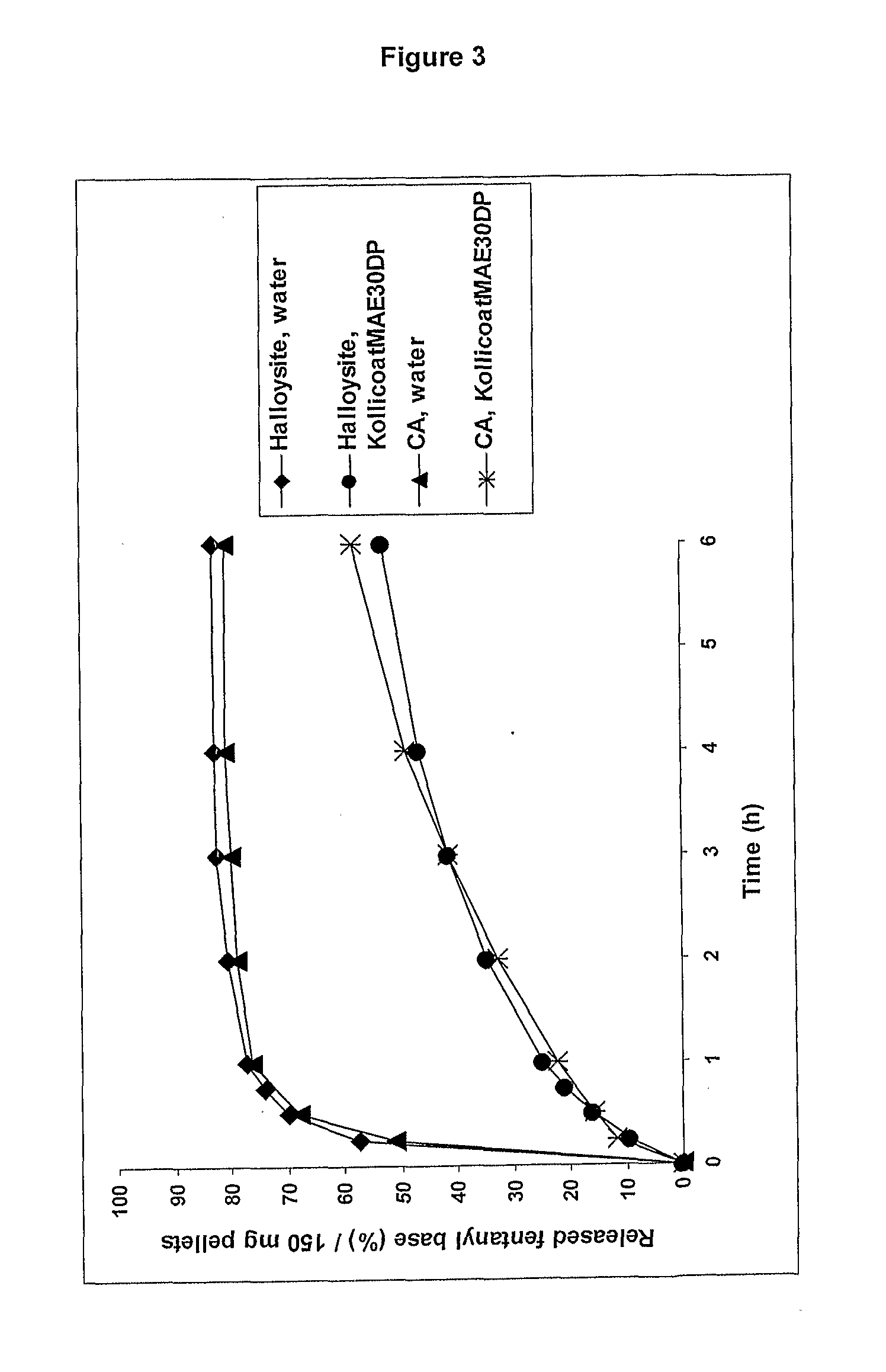
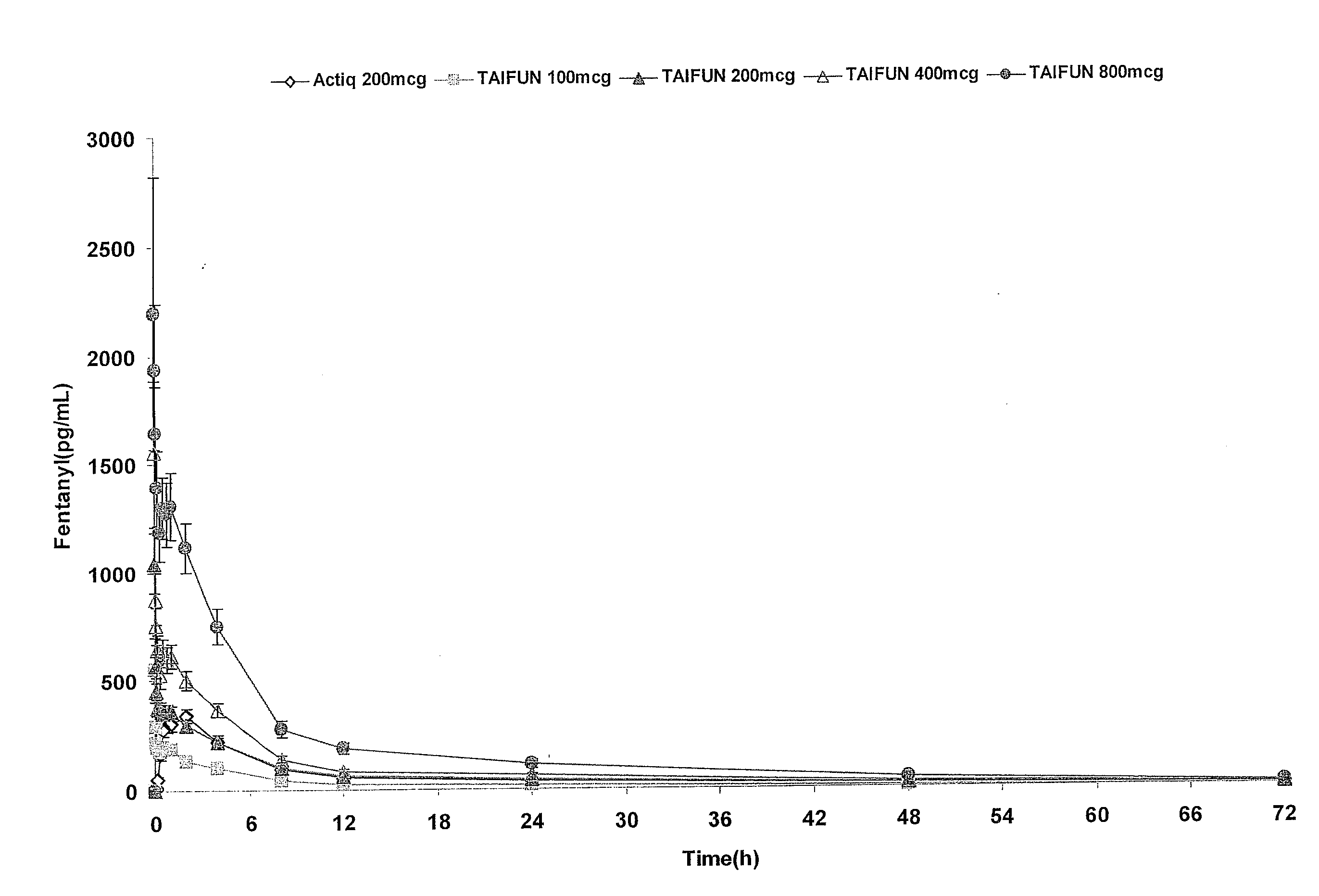
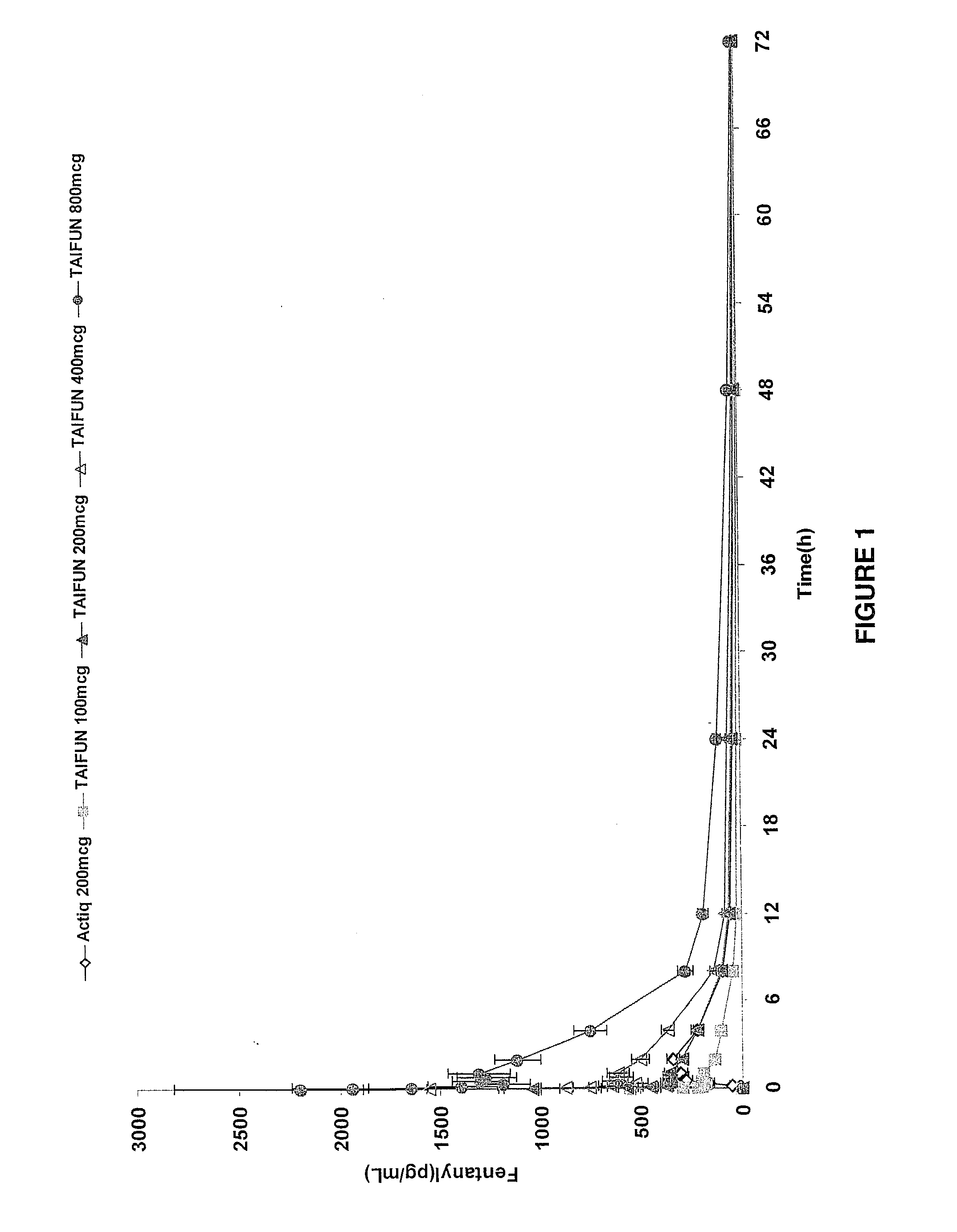
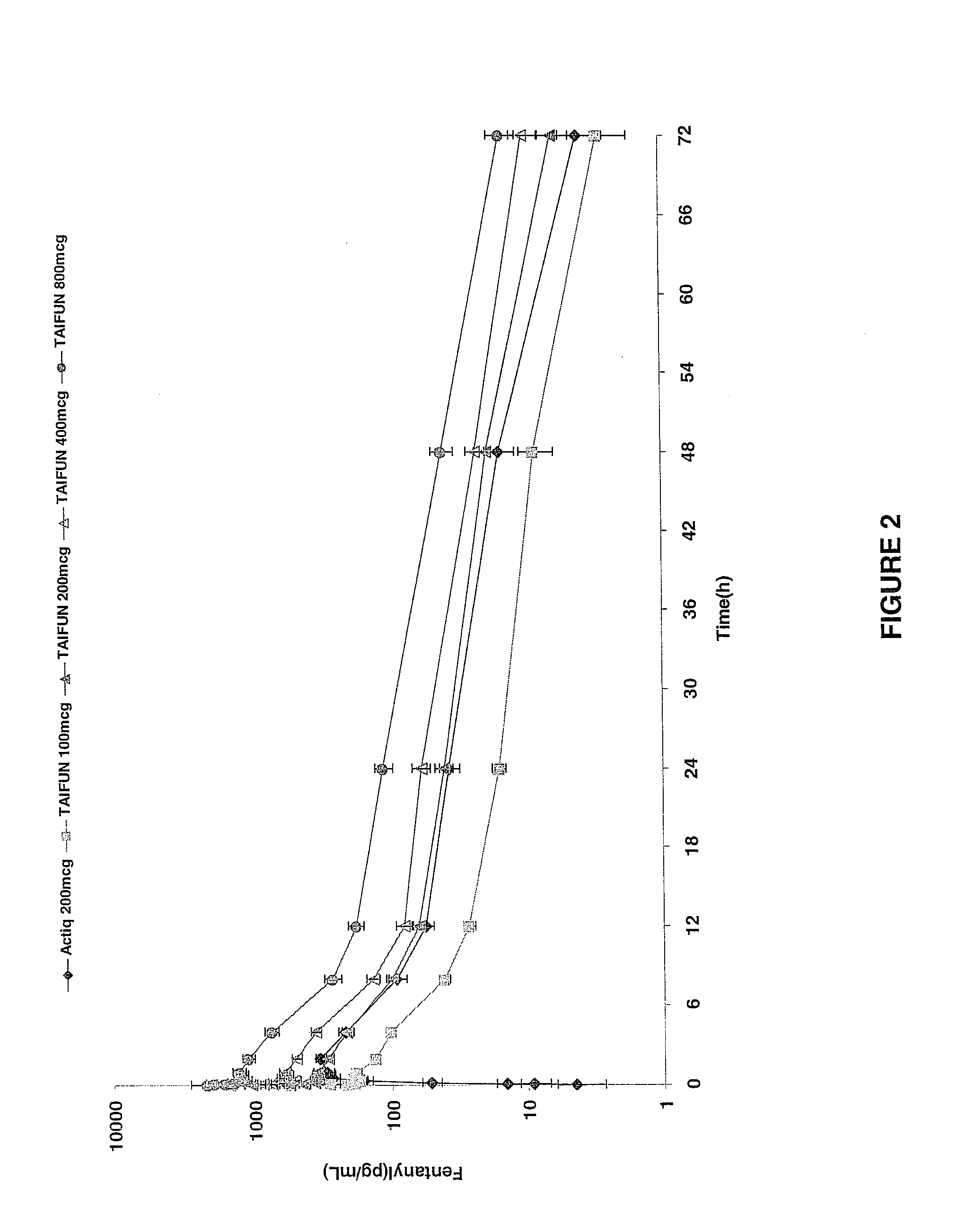
InactiveUS20090011030A1Treatment and alleviation of painAct quicklyBiocidePowder deliveryPulmonary inhalationFentanyl
The present invention is directed to a powdered formulation comprising an analgesic, preferably fentanyl, for use in pulmonary inhalation administration for the rapid analgesic titration of pain, in particular breakthrough pain. Upon administration, the powdered formulation is able to provide a narrower titration range in patients suffering from pain, as well as effective analgesic amounts of fentanyl in a shorter time and at lower dose levels of administered fentanyl when compared to fentanyl administered by an oral transmucosal route.
Owner:LAB INT
Beta Amino Acid Derivatives as Integrin Antagonists
ActiveUS20140038910A1Sufficient amountToxic reductionBiocideUrea derivatives preparationIntegrin antagonistFibrosis
Disclosed herein are novel pharmaceutical agents which are useful as integrin receptor antagonists that mediate the pathologic processes of angiogenesis and fibrosis and as such are useful in pharmaceutical compositions and in methods for treating conditions mediated by these integrins by inhibiting or antagonizing these integrins. The novel pharmaceutical agents include those of the formula:wherein the variables are defined herein. Also provided are pharmaceutical compositions, kits and articles of manufacture comprising such pharmaceutical agents. Methods and intermediates useful for making the pharmaceutical agents and methods of using the pharmaceutical agents are also provided.
Owner:SAINT LOUIS UNIVERSITY
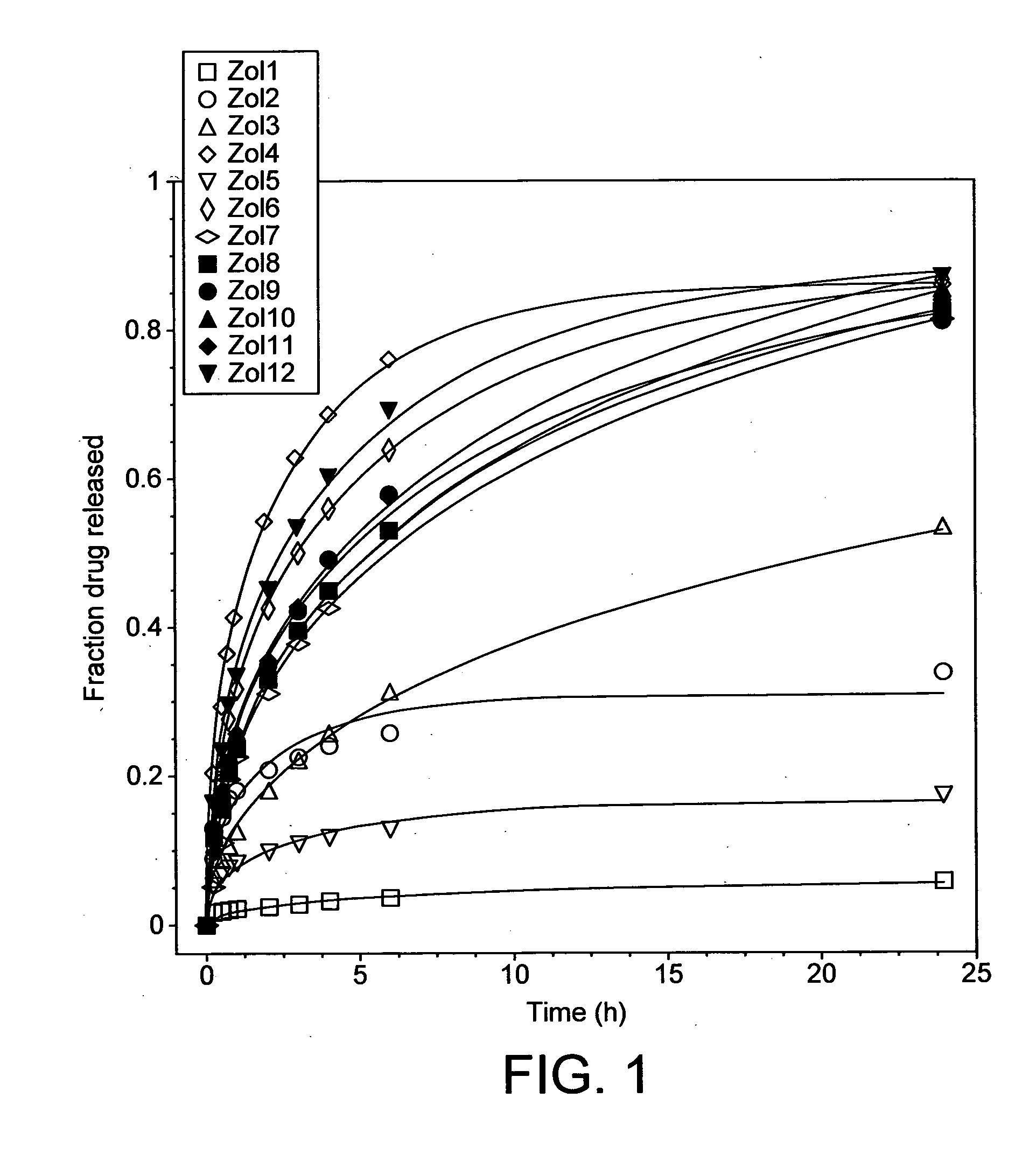
Controlled release pharmaceutical composition
InactiveUS20130171199A1Reduce dosing frequencyLonger actingBiocideDispersion deliveryControlled releaseExcipient
An oral controlled release pharmaceutical composition comprising a core and a polymer dispersion and its preparation method are disclosed. The core is selected from the group consisting of ‘the drug coated core’ and ‘the drug loaded core’. The drug coated core comprises an inert excipient based sphere and a coat of drug composition. The drug loaded core comprises at least a drug, a binder and at least one pharmaceutically acceptable excipient. The polymer dispersion used to coat the core comprises at least one controlled release polymer and at least one pharmaceutically acceptable excipient. The oral controlled release pharmaceutical composition further comprises an in-situ gelling system comprising at least one gelling polymer.
Owner:ABBOTT HEALHCARE PROD BV
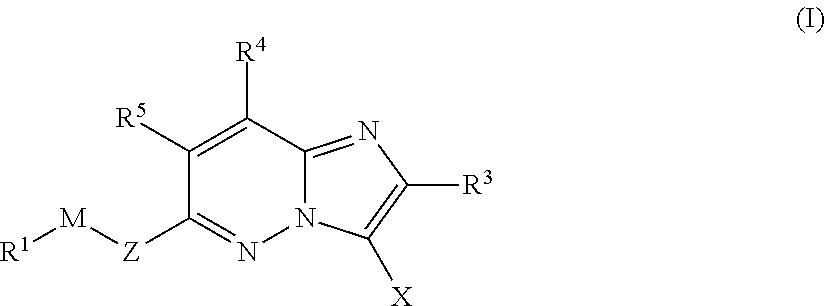
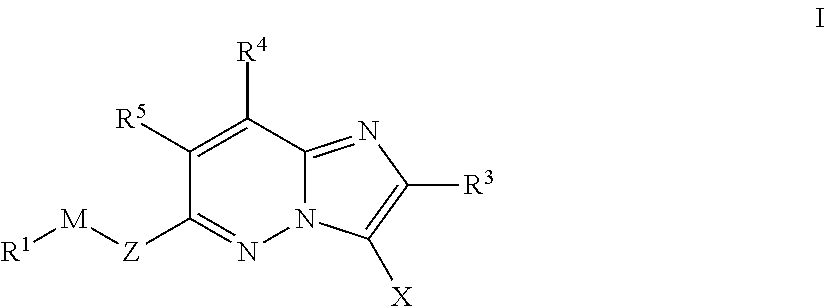
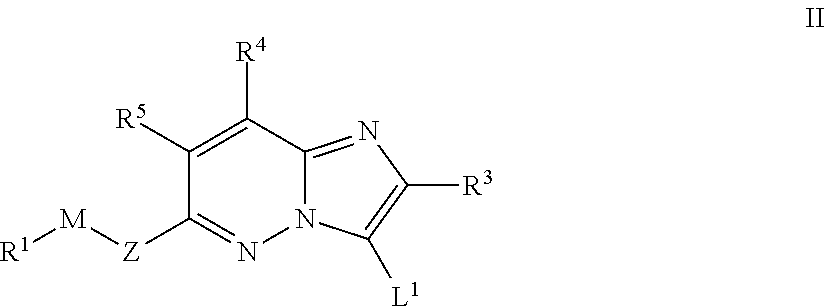
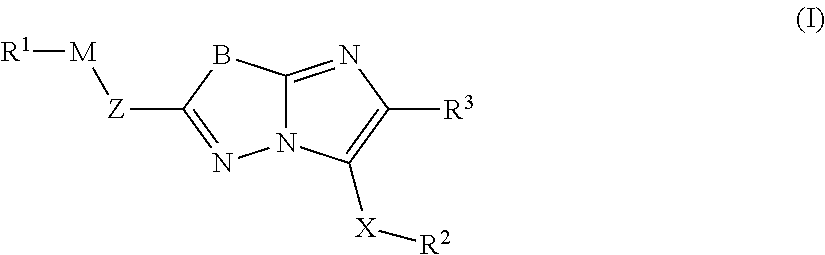
Imidazopyridazines for Use as Protein Kinase Inhibitors
InactiveUS20110046127A1Easy to convertToxic reductionBiocideOrganic chemistryPTK InhibitorsIsrapafant
There is provided compounds of formula (I): wherein Z, M, R1, X, R3, R4 and R5 have meanings given in the description, an pharmaceutically-acceptable esters, amides, solvates or salts thereof, which compounds are useful in the treatment of diseases in which inhibition of a protein kinase (e.g. a PIM family kinase or PI3-K) is desired and / or required, an particularly in the treatment of cancer.
Owner:FUNDACION CENT NACIONAL DE INVESTIGACIONES ONCOLOGICAS CARLOS III
New compounds
Owner:BOEHRINGER INGELHEIM INT GMBH
Inhibitor of p38 map kinase
ActiveUS9447076B2Toxic reductionLonger actingOrganic active ingredientsPowder deliveryMedicineKinase
A compound has the following formula:The compound is a p38 MAP kinase inhibitor. The compound and its pharmaceutically acceptable salts can be used for treatment of conditions, such as inflammatory diseases.
Owner:RESPIVERT
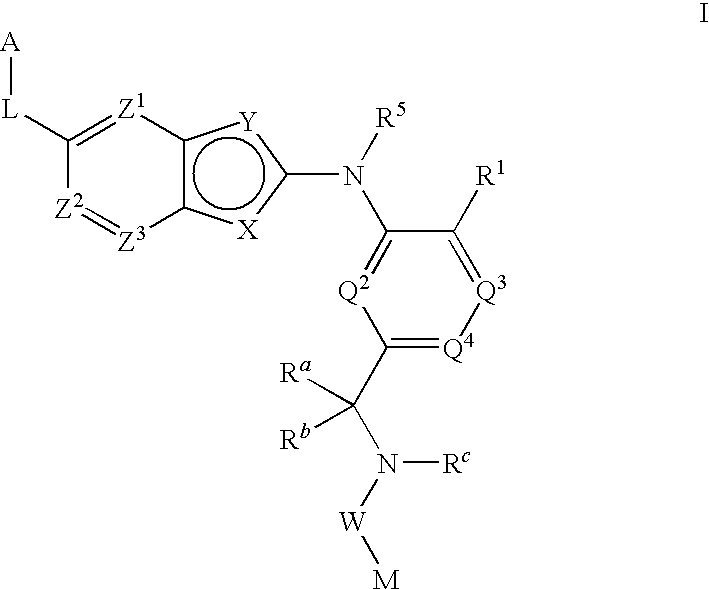
Bis-aromatic compounds useful in the treatment of inflammation
There is provided compounds of formula (I): wherein Y, ring A, D1, D2, D3, L1, Y1, L2, Y2, L3 and Y3 have meanings given in the description, and pharmaceutically-acceptable salts thereof, which compounds are useful in the treatment of diseases in which inhibition of leukotriene C4 synthase is desired and / or required, and particularly in the treatment of a respiratory disorder and / or inflammation.
Owner:BIOLIPOX AB
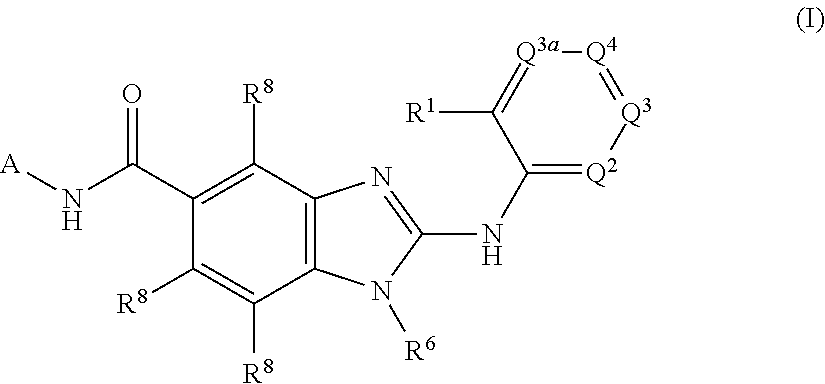
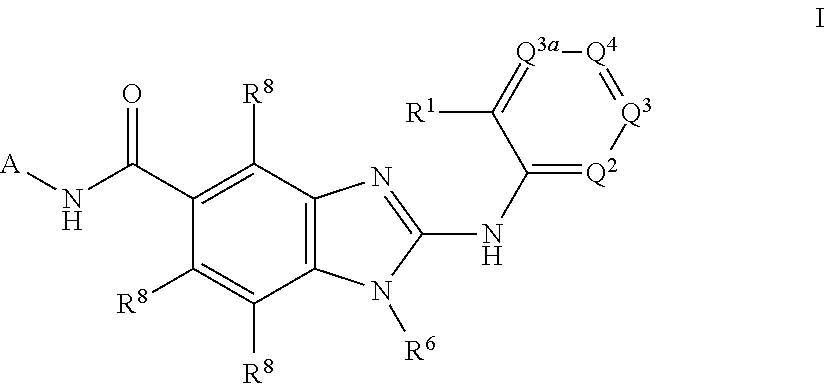
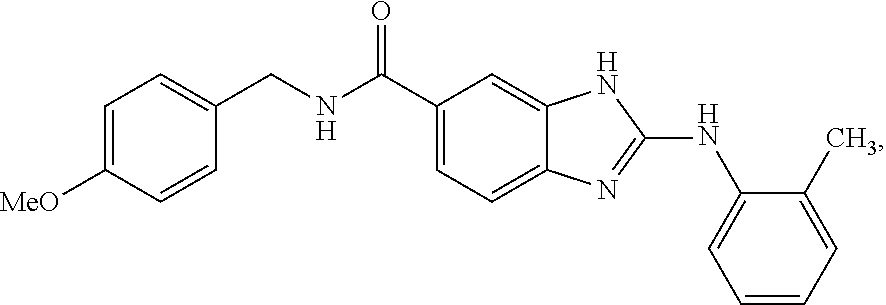
1H-Benz Imidazole-5-Carboxamides As Anti-Inflammatory Agents
InactiveUS20110312935A1Reduce formationMore efficaciousBiocideSenses disorderDiseasePharmaceutical medicine
Owner:OREXO AB
Transdermal drug administration device
ActiveUS20130273119A1Prevent extractionLonger actingBiocideNervous disorderDrug administrationCarrier material
A transdermal drug administration device comprising a drug delivery element (10) defining a contact surface (12) for location, in use, against a patient's skin. The drug delivery element (10) includes a sustained-release pharmaceutical composition. The composition comprises a network of a carrier material having a high mechanical strength and an active pharmaceutical ingredient. The active pharmaceutical ingredient is co-formedly interspersed within pores in the solid, continuous network of the carrier material.
Owner:EMPLICURE AB
New compounds
Owner:BOEHRINGER INGELHEIM INT GMBH
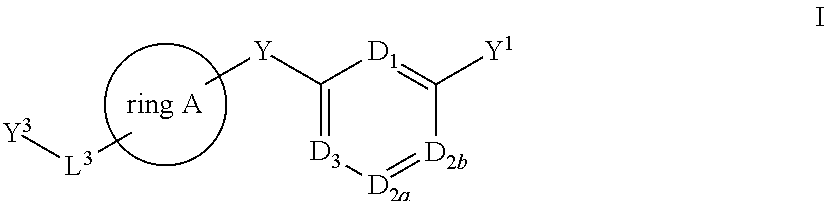
Bis-aryl compounds for use as medicaments
There is provided compounds of formula I, wherein ring A, D1, D2a, D2b, D3, L1, Y1, L3 and Y3 have meanings given in the description, and pharmaceutically-acceptable salts thereof, which compounds are useful in the treatment of diseases in which inhibition of leukotriene C4 synthase is desired and / or required, and particularly in the treatment of a respiratory disorder and / or inflammation.
Owner:BIOLIPOX AB
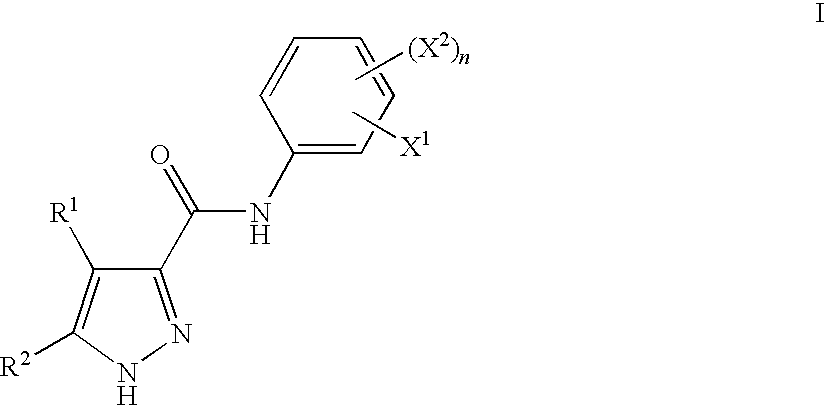
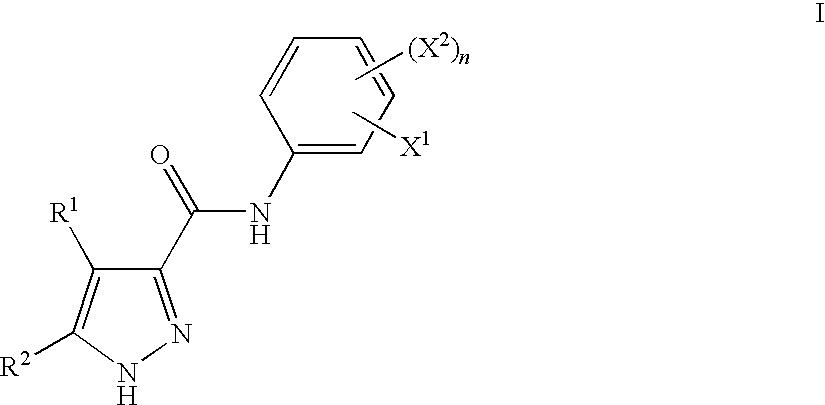
Pyrazoles Useful in the Treatment of Inflammation
InactiveUS20090143455A1Improve oral bioavailabilityReduce gapBiocideOrganic active ingredientsDiseaseLipoxygenase activity
There is provided compounds of formula I,wherein R1, R2, X1, X2 and n have meanings given in the description, and pharmaceutically-acceptable salts thereof, which compounds are useful in the treatment of diseases in which inhibition of the activity of a lipoxygenase (e.g. 15-lipoxygenase) is desired and / or required, and particularly in the treatment of inflammation.
Owner:BIOLIPOX AB
N-myristoyl transferase inhibitors
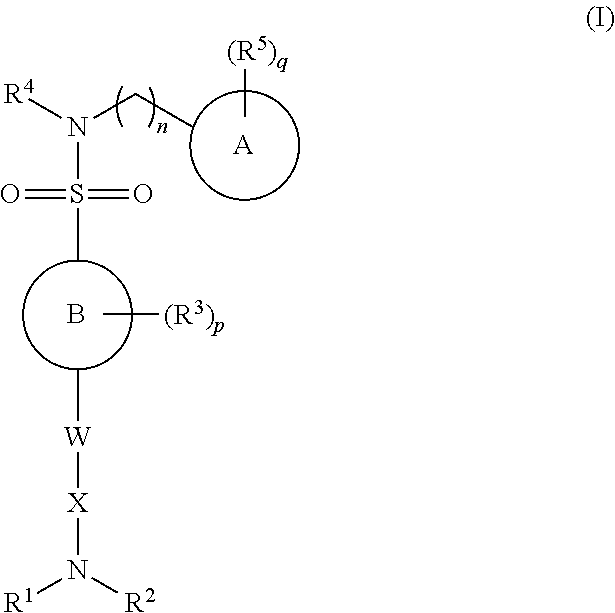
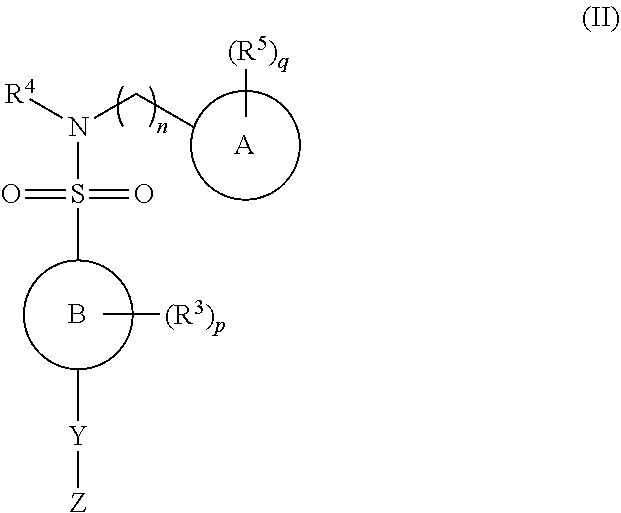
ActiveUS9156811B2Improve permeabilityRapid onset of activityBiocideAntimycoticsN-myristoyltransferaseBiochemistry
The present invention relates to N-heterocyclic sulphonamide compounds, in particular pyrazole sulphonamide compounds, and their use as N-myristoyl transferase inhibitors.
Owner:UNIVERSITY OF DUNDEE
Water Injection Device for an Internal Combustion Engine of a Motor Vehicle
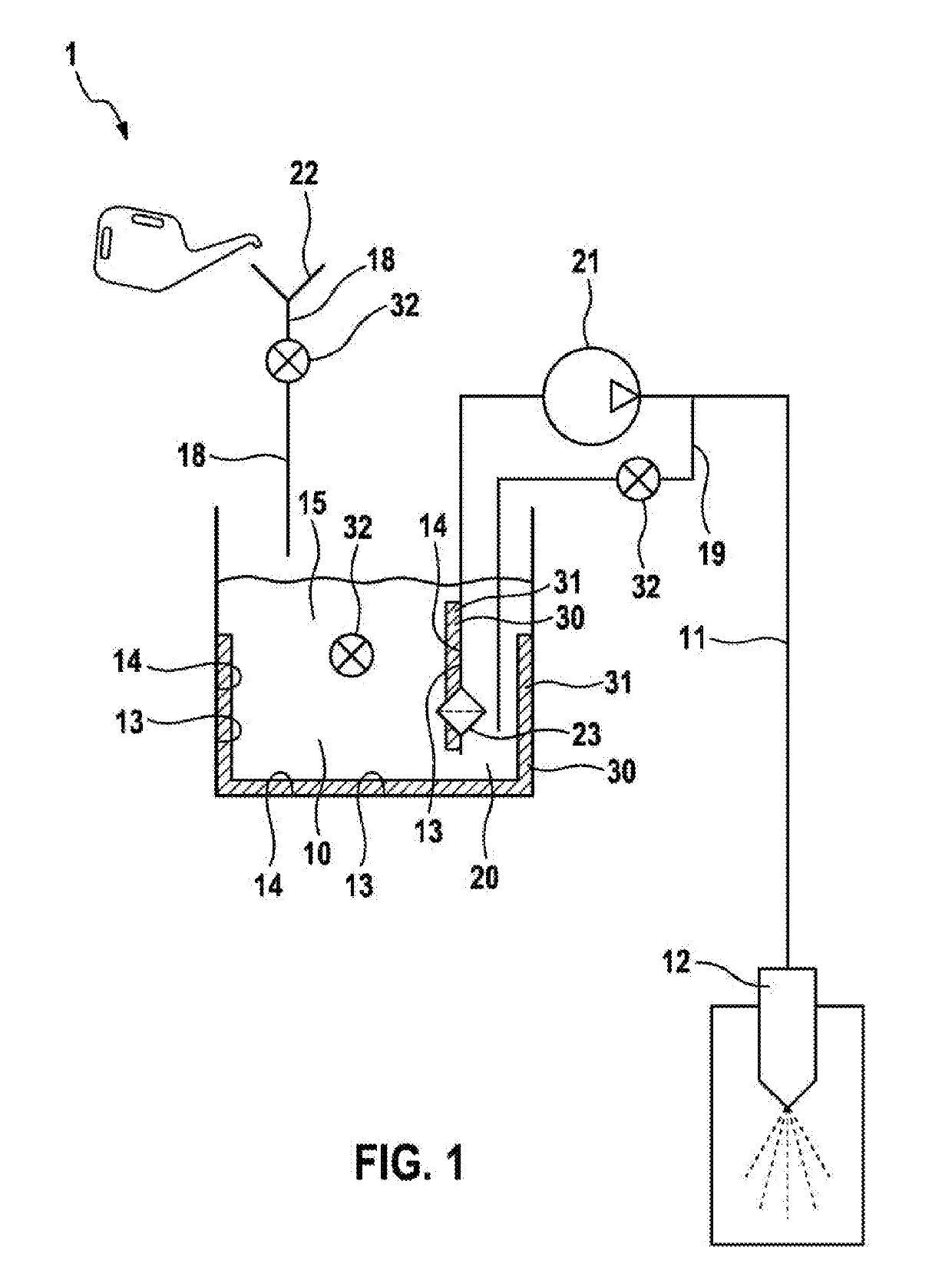
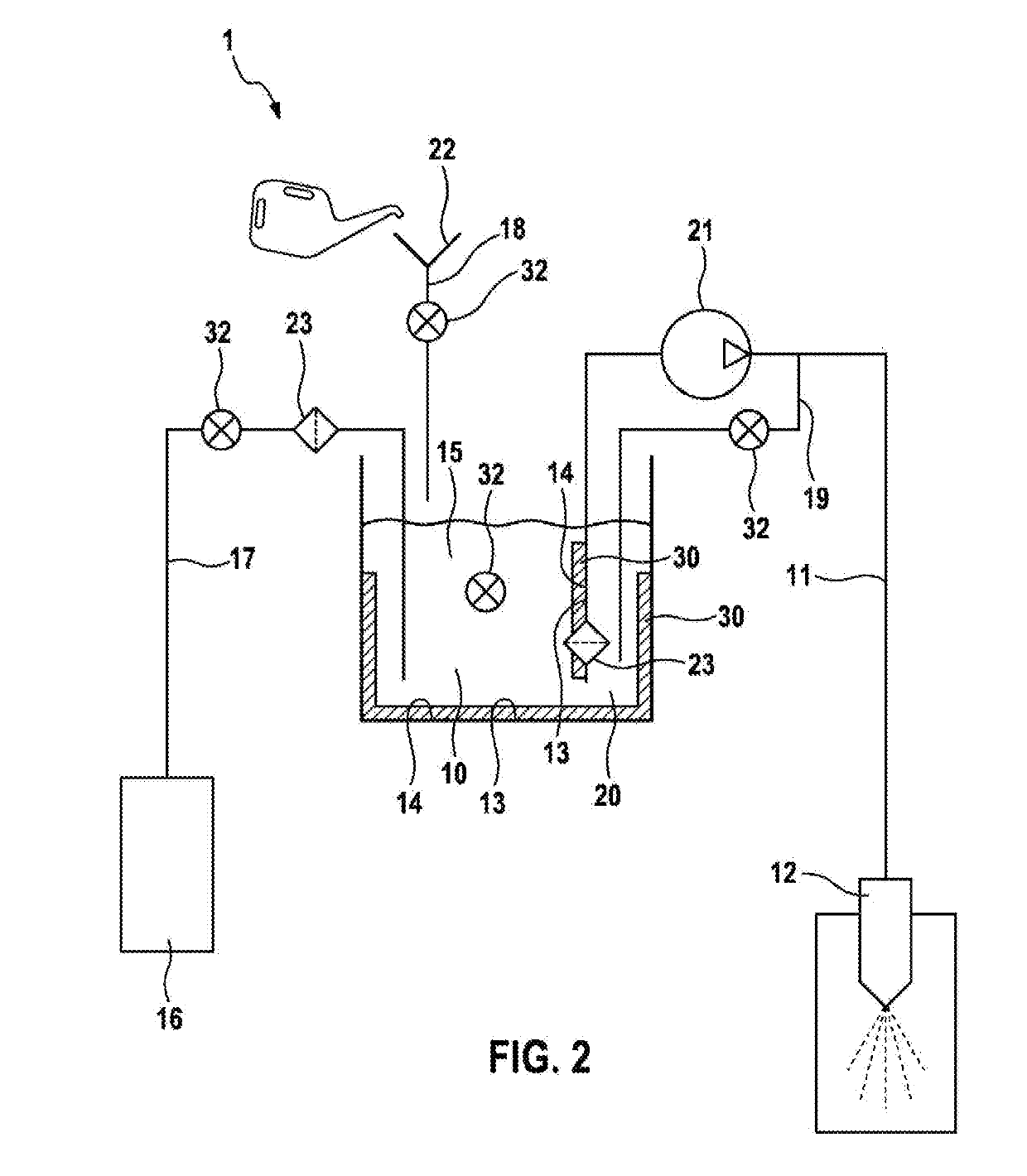
InactiveUS20190257270A1Little biocontaminationLonger actingInternal combustion piston enginesNon-fuel substance addition to fuelExternal combustion engineInternal combustion engine
A water injection device for an internal combustion engine of a motor vehicle includes at least one water injector for injecting water into an internal combustion engine. The water injection device further includes a water tank and a first supply line. The first supply line is configured to connect the water tank to the water injector. At least one biocide for controlling harmful organisms in the water injection device is provided in the water injection device.
Owner:ROBERT BOSCH GMBH
Bis Aromatic Compounds for Use in the Treatment of Inflammation
There is provided compounds of formula (I), wherein ring A, D1, D2a, D2b, D3, Y, Y1, L3 and Y3 have meanings given in the description, and pharmaceutically-acceptable salts thereof, which compounds are useful in the treatment of diseases in which inhibition of leukotriene C4 synthase is desired and / or required, and particularly in the treatment of a respiratory disorder and / or inflammation.
Owner:BIOLIPOX AB
Composition for sustained drug delivery comprising geopolymeric binder
ActiveUS20120252845A1Low risk of and diversionSustained releaseBiocideNervous disorderDrug deliveryGeopolymer
Owner:EMPLICURE AB
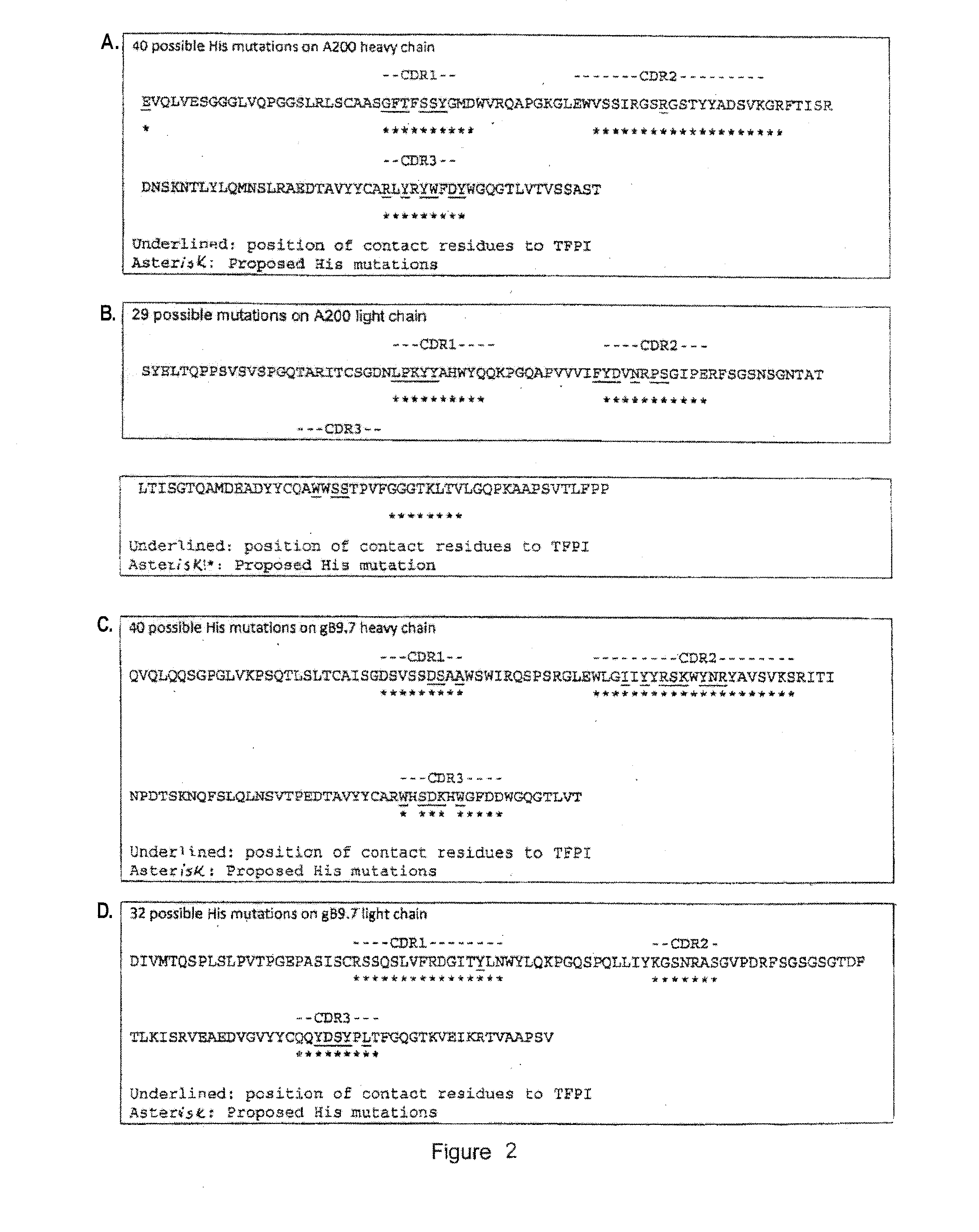
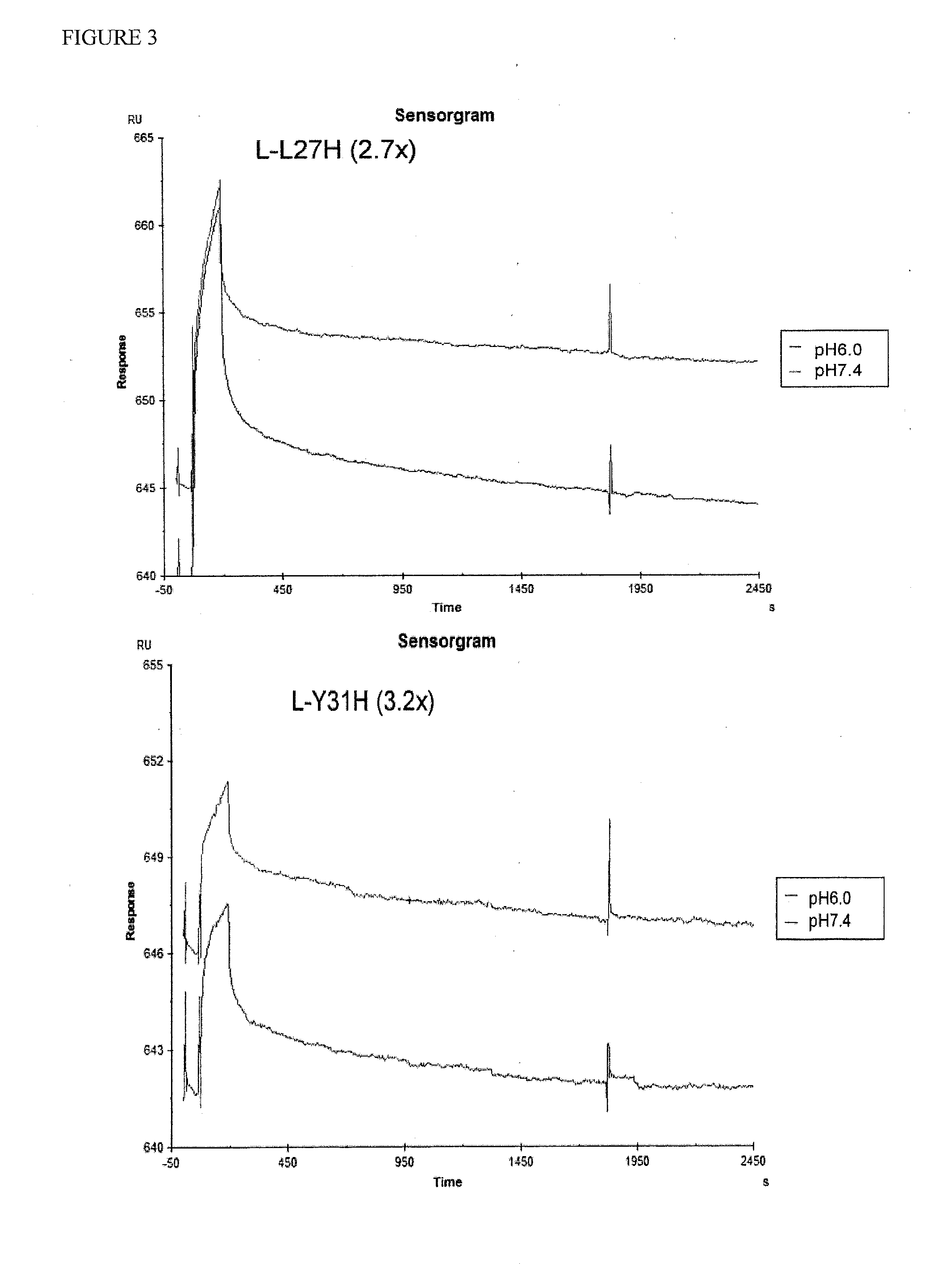
Anti-tfpi antibody variants with differential binding across ph range for improved pharmacokinetics
ActiveUS20140275493A1Extended half-lifeReduce the burden onImmunoglobulins against animals/humansFermentationAntigenLow affinity
Antibodies are disclosed that bind to and inhibit the anti-coagulant function of TFPI and have a lower affinity for TFPI at pH 6.0 than at pH 7.4. The lower affinity at pH 6 improves circulating half-life (T½) due to reduced target mediated clearance, a process by which an antibody / antigen complex is endocytosed and trafficked to the lysosome where both components are degraded. The lower affinity at pH 6.0 results in disruption of the complex prior to lysosome targeting and allows for re-circulation of the antibody. Specific modifications to antibody binding by histidine residue substitution are disclosed along with methods of use.
Owner:BAYER HEALTHCARE LLC
Positive modulators of nicotinic receptor agonists
InactiveUS20050245595A1Enhance efficacy of agonistRestore normal interneuronal communicationBiocideNervous disorderEnantiomerNicotinic Receptor Agonist
Owner:ASTRAZENECA AB
Selective etching of a matrix comprising silver NANO wires
InactiveUS20140291287A1Improve throughputLess expensiveDecorative surface effectsSolid-state devicesEtchingCarbon nanotube
The present invention refers to a method for selectively structuring of a polymer matrix comprising AgNW (silver nano wires) or CNTs (carbon nano tubes) or comprising mixtures of AgNW and CNTs on a flexible plastic substructure or solid glass sheet. The method also includes a suitable etching composition, which allows to proceed the method in a mass production.
Owner:MERCK PATENT GMBH
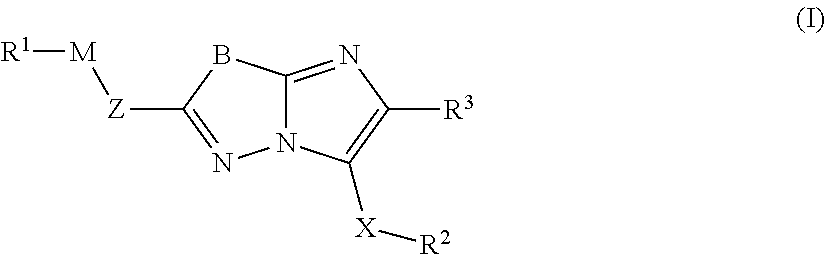
Imidazolothiadiazoles for use as protein kinase inhibitors
There is provided compounds of formula (I), wherein Z, M, R1, X, R2, R3 and B have meanings given in the description, and pharmaceutically-acceptable esters, amides, solvates or salts thereof, which compounds are useful in the treatment of diseases in which inhibition of a protein kinase (e.g. a PIM family kinase or PI3-K) is desired and / or required, and particularly in the treatment of cancer.
Owner:FUNDACION CENT NACIONAL DE INVESTIGACIONES ONCOLOGICAS CARLOS III
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com