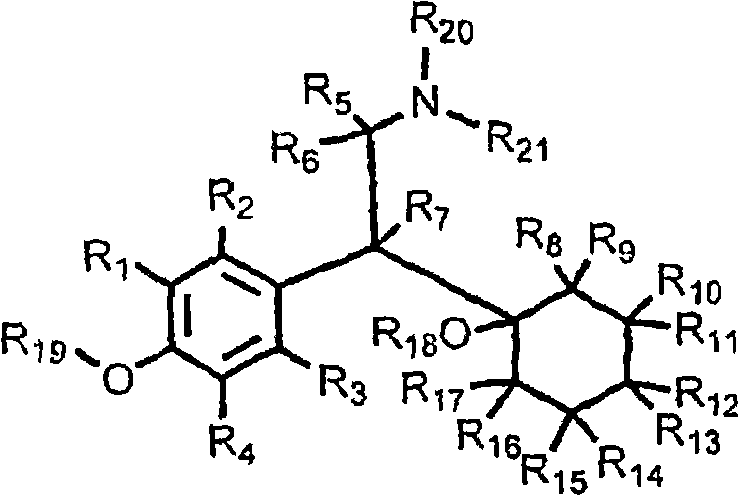
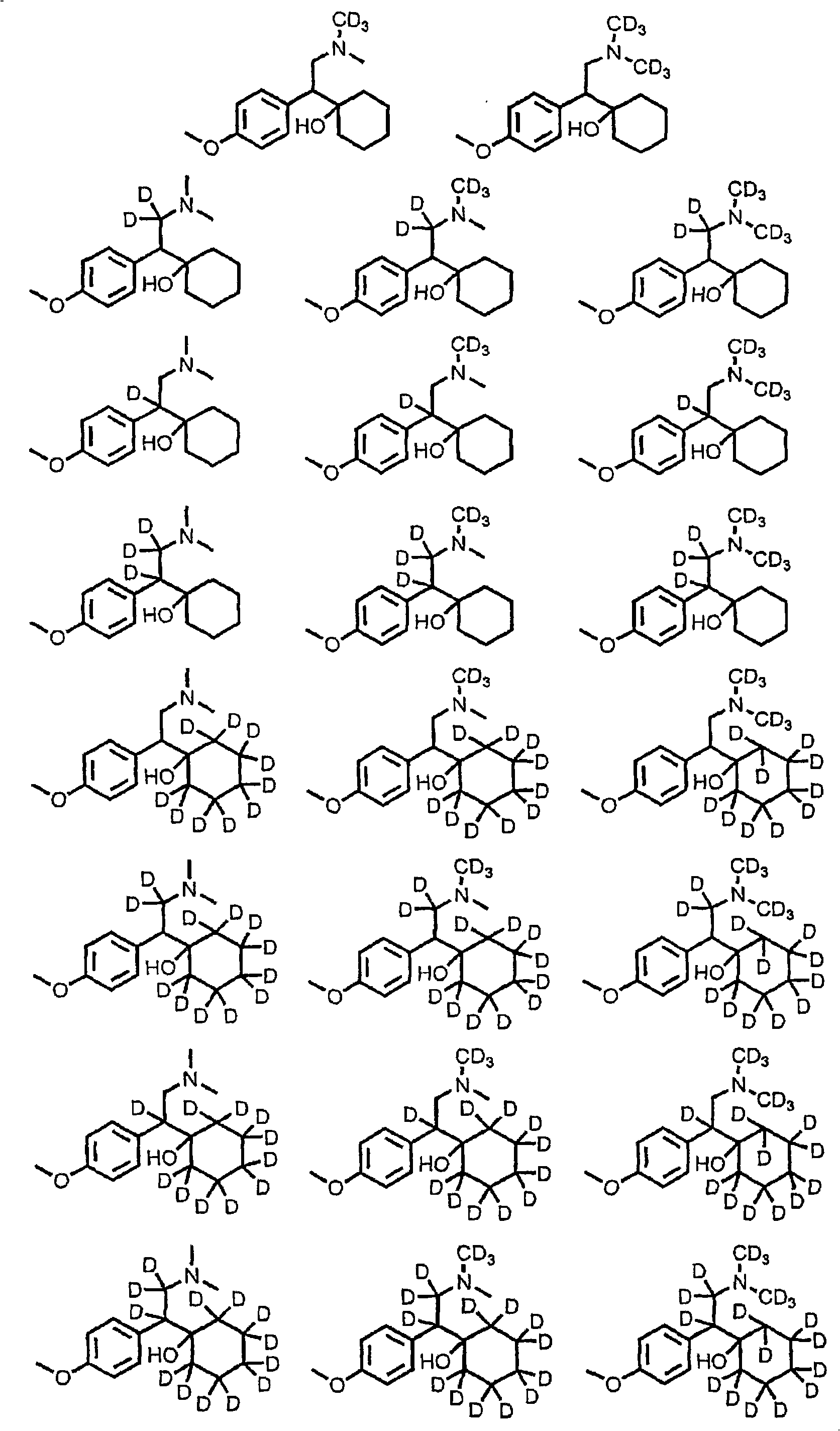
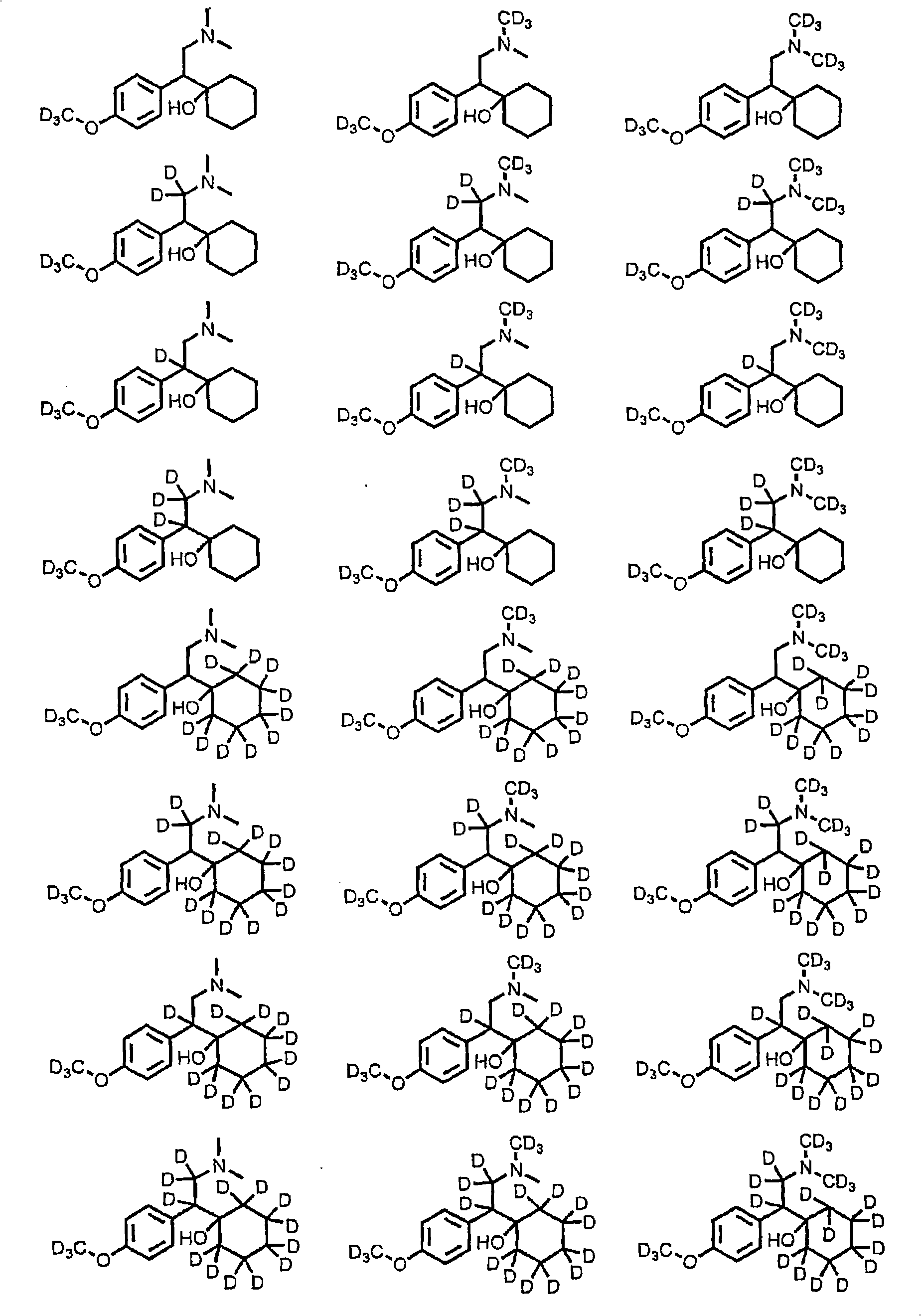
Substituted phenethylamines with serotoninergic and/or norepinephrinergic activity
A technology of isotope enrichment and enantiomers, applied in organic active ingredients, medical preparations containing active ingredients, organic chemistry, etc., can solve problems such as noncompliance, short half-life, drug withdrawal, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179] According to the method of known literature (Ouk et al., Green Chemistry, 2002, 4(5), 431-435, which is incorporated herein by reference), by making d 6 -(4-Hydroxyphenyl)-acetic acid (1 equivalent, Cambridge Isotopes Laboratories), K 2 CO 3 (0.04 equiv) and d 6 - Dimethyl carbonate (1.25 equivalents, Cambridge Isotopes Laboratories) was reacted at 160°C until complete to prepare d 9 -(4-Methoxyphenyl)-acetic acid.
Embodiment 2
[0180] The title compound was prepared by the method described by Yardley et al. in Journal of Medicinal Chemistry 1990, 33(10), 2899-2905, which is hereby incorporated by reference in its entirety. will d 9 -(4-Methoxyphenyl)-acetic acid (1 equiv) in dichloromethane was treated with oxalyl chloride (1.22 equiv) and DMF (catalytic amount) and stirred at room temperature until all the acid was converted to acid chloride. The solvent was removed under reduced pressure, and the residue was dissolved in dichloromethane and washed with d 6 - Treatment with dimethylamine hydrochloride (1 equiv, Cambridge Isotopes Laboratories), ethyldiisopropylamine (2.1 equiv) and DMAP (0.2 equiv). The mixture was stirred overnight. The solvent was removed under reduced pressure and the crude residue was purified by silica gel column chromatography.
Embodiment 3
[0181] The title compound was prepared according to the method described by Yardley et al. in Journal of Medicinal Chemistry 1990, 33(10), 2899-2905. d at -78°C 15 - 2-(4-Methoxyphenyl)-N,N-dimethyl-acetamide (1 eq) in THF was treated with n-butyllithium (1 eq). The mixture was stirred at -78 °C for 90 min; d was added 10- Cyclohexanone (1.2 equivalents, Sigma-Aldrich) in THF, maintaining stirring until completion. By joining D 2 O (2 equiv) quenched the reaction, the mixture was warmed to room temperature, the solvent was removed under reduced pressure, and the crude residue was purified by silica gel column chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com