Method for synthesizing mirtazapine
A synthetic method and compound technology, applied in the direction of organic chemistry, can solve the problems of many by-products, expensive raw materials, harsh conditions, etc., and achieve the effects of easy control, simple operation, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
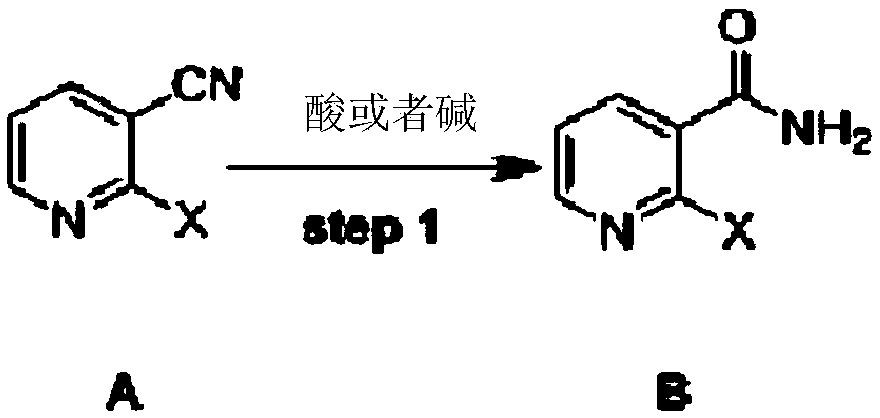
[0037] Example 1: Preparation of 2-chloronicotinamide (Compound B)
[0038] Add 400ml of concentrated sulfuric acid and 2-chloronicotinonitrile (138g, 1mol) to a 1000ml three-necked flask. After it is completely dissolved, heat to 90°C and stir for 2 hours. Pour the above reaction solution into a mixture of 1000ml ammonia and 1kg ice After stirring for 1 hour, a solid crude product was obtained after filtration. The crude product was added to 1000 ml of ethyl acetate, stirred for 1 hour, and separated by filtration to obtain a white solid. The white solid was further dried by blowing at 50° C. to obtain 153 g of intermediate 2-chloronicotinamide as a white solid with a yield of 98%. 1HNMR (300M, DMSO) δ 8.46 (dd, 1H), 8.06 (s, 1H), 7.91 (dd, 1H), 7.77 (S, 1H) 7.48 (dd, 1H); 13CNMR (75M, DMSO) δ 167. 11, 150.20, 146.48, 138.00, 133.53, 123.18
Embodiment 2
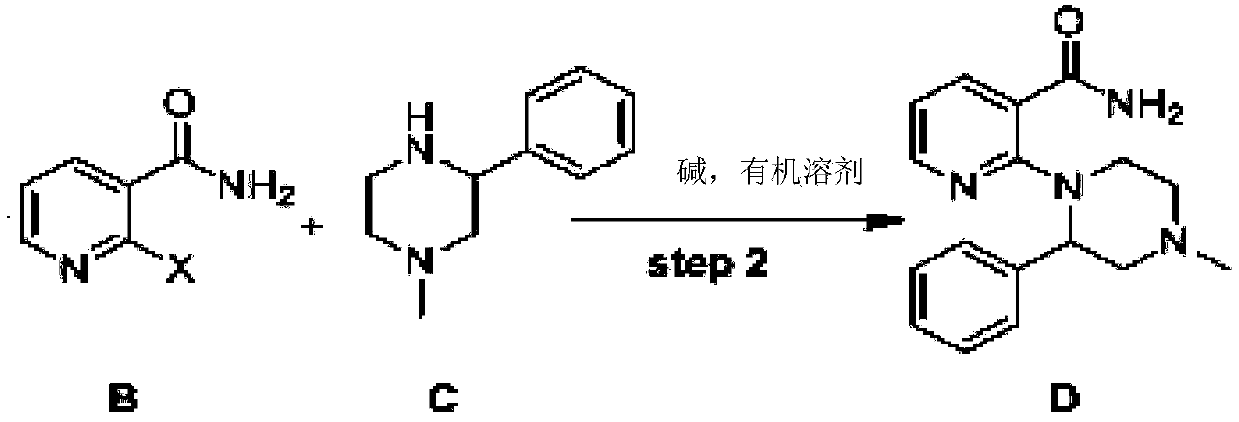
[0039] Example 2: Preparation of 2-(4-methyl-2-phenyl-1-piperazinyl)nicotinamide (Compound D)
[0040] Add 600ml of N,N-dimethylformamide, 2-chloronicotinamide (153g, 0.98mol), 1-methyl-3phenylpiperazine (172g, 0.98mol), potassium fluoride to a 1000ml three-necked flask (114g, 1.96mol), heated to 140°C, stirred for 16 hours, added the crude product to 1000ml of ice water, stirred for 1 hour, filtered and separated to obtain a white solid. The white solid was further dried by blowing at 50°C to obtain 261 g of 2-(4-methyl-2phenyl-1-piperazinyl)nicotinamide, a white solid, with a yield of 90%. 1HNMR (300M, DMSO) δ 8.40 (dd, 1H), 7.88 (dd, 1H), 7.56 (s, 1H), 7.50 (s, 1H), 7.49-7.36 (m, 2H), 7.32-7.28 (m , 3H), 6.86(dd, 1H), 4.73-4.64(m, 2H), 3.19-3.10(m, 1H), 3.05-2.98(m, 3H), 2.52-2.41(m, 2H), 2.29(s , 3H); 13CNMR (75M, DMSO) δ 168.11, 162.50, 146.60, 140.00, 137.30, 129.01, 128.50, 127.93, 122.82, 121.30, 54.93, 52.36, 54.68, 53.62, 45.90
Embodiment 3
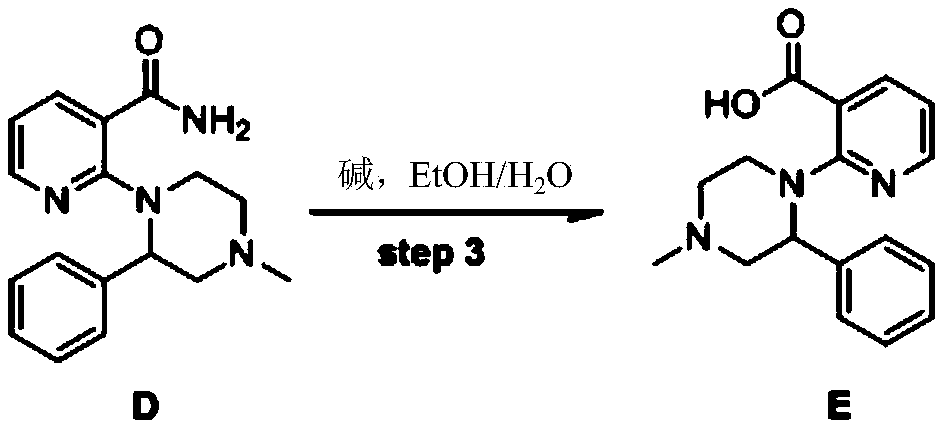
[0041] Example 3: Preparation of 2-(4-methyl-2phenyl-1-piperazinyl)nicotinic acid (Compound E)
[0042] Add 600ml of 95% ethanol, 2-(4-methyl-2phenyl-1-piperazinyl)nicotinamide (148g, 0.5mol), potassium hydroxide (280g, 5mol) to a 1000ml three-necked flask, and heat to increase Bring to 110℃, stir and reflux for 26 hours, add 2L of water, remove the solvent under reduced pressure, add dropwise 6N hydrochloric acid to pH 7-8, extract three times with dichloromethane (2L×3), and dry the organic phase with 1kg anhydrous sodium sulfate , A white solid was obtained. The white solid was further dried by blowing at 50°C to obtain 134 g of 2-(4-methyl-2phenyl-1-piperazinyl)nicotinic acid, a white solid, with a yield of 90%. 1HNMR (300M, CDCl3) δ 8.50 (dd, 1H), 8.31 (dd, 1H), 7.26-7.22 (m, 2H), 7.18-7.06 (m, 4H), 4.80 (dd, 1H), 3.44 (td , 1H), 3.17-3.06 (m, 3H), 2.63-2.53 (m, 2H), 2.43 (s, 3H); 13CNMR (75M, CDCl3) δ 165.31, 159.86, 152.22, 140.29, 136.97, 128.29, 128.02 , 122.60, 121.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com