Method for preparing antidepressant mirtazapine
A technology of mirtazapine and antidepressant, which is applied in the field of preparation of antidepressant drug mirtazapine, and can solve the problems of increasing synthesis steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
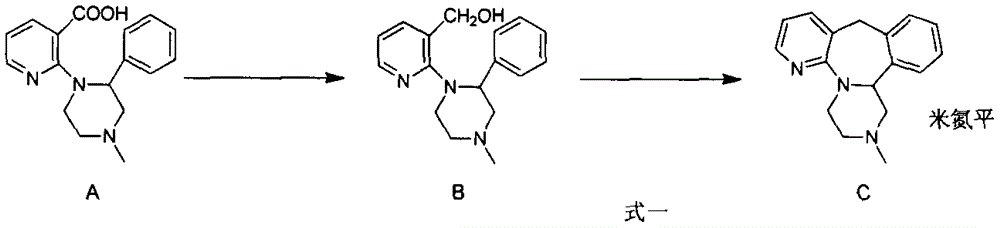
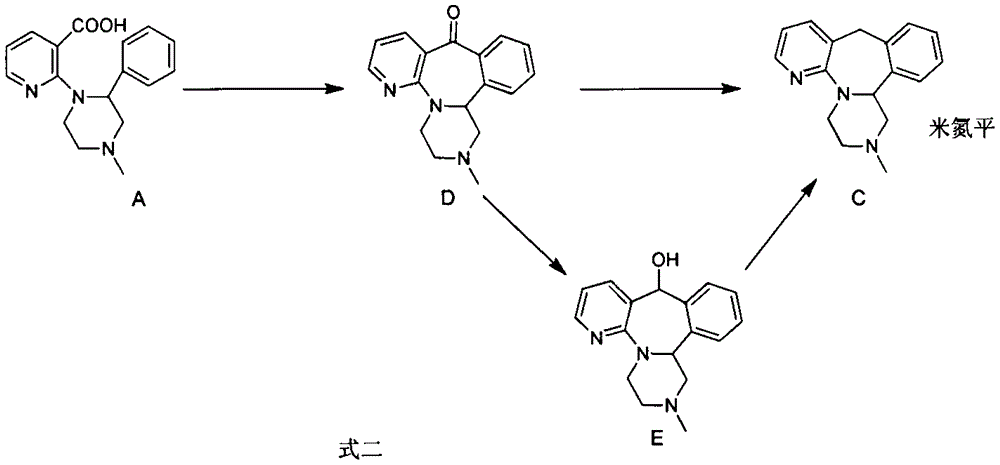
[0021] Example 1: 1,3,4,14b-tetrahydro-2-methylpyrazine[2,1-a]pyrido[2,3-c][2]benzazepine-10(2H) - Preparation of ketones
[0022] Under a nitrogen atmosphere, 1-(3-carboxy-2-pyridyl)-2-phenyl-4-methylpiperazine (5.0 g, 16.8 mmol), dichloromethane (40 mL) were added to a 100 mL three-necked flask, N,N-Dimethylformamide (1mL) was stirred, and then the system was cooled to about 0°C with an ice-water bath, and thionyl chloride (4.0g, 33.6mmol) was added dropwise into the reaction system liquid, and in 15min finished adding. After removing the ice-water bath and reacting at room temperature for 70 minutes, the system liquid was cooled to about 0°C, and anhydrous aluminum trichloride (12.1 g, 90.6 mmol) was added in batches, and after the addition was completed, the temperature was naturally raised to room temperature for reaction, and TLC It was detected that the reaction of raw materials was complete. Pour the reaction solution into ice water (200 mL), add 10% NaOH aqueous so...
Embodiment 2
[0024] Embodiment 2: the preparation of mirtazapine
[0025] Under a nitrogen atmosphere, add D (419.0 mg, 1.5 mmol), diethylene glycol (8 mL), and dimethyl sulfoxide (1 mL) into a 25 mL three-necked flask and stir. Then add solid potassium hydroxide (210.4mg, 3.8mmol), then weigh 80% hydrazine hydrate (375.5mg, 6.0mmol) with a syringe and add it dropwise to the reaction system. After the temperature is stable, heat the system to 120-125°C for 1 hour, then distill off the water and excess hydrazine hydrate in the system under reduced pressure. After cooling, pour the system solution into water (25 mL) and stir for 20 min, extract three times with ethyl acetate (50 mL), combine the organic phases, wash once with water, and dry over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness to obtain 378.1 mg of a brown-yellow solid. After purification by column chromatography (ethyl acetate:petroleum ether:triethylamine=10:40:0.05), 351.1 mg of a whit...
Embodiment 3
[0027] Embodiment 3: the preparation of mirtazapine
[0028] Under nitrogen atmosphere, add D (2.8g, 10.0mmol) and diethylene glycol (25mL) into a 50mL three-necked flask and stir. Then add potassium hydroxide solid (1.4g, 25.0mmol), then weigh 80% hydrazine hydrate (2.5g, 40.0mmol) in the dropping funnel, add dropwise in the reaction system, the reaction exotherm is obvious, after adding Raise the temperature by 8°C in total. After the temperature stabilizes, heat the system to 120-125°C and react for 1 hour. Then evaporate the water and excess hydrazine hydrate in the system under reduced pressure. After steaming, continue to heat up to 170°C for reaction. The response is complete. After cooling, the system solution was poured into water (50 mL) and stirred for 20 min, extracted three times with ethyl acetate (120 mL), the organic phases were combined, washed once with water, and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to drynes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com