Intra-oral disintegration compositions and process for producing same
A technology of composition and disintegrant, which is applied in the field of oral disintegration composition, can solve the problems of high cost and slow dissolution, achieve the effect of skilled workers, improve curative effect, and save R&D and production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
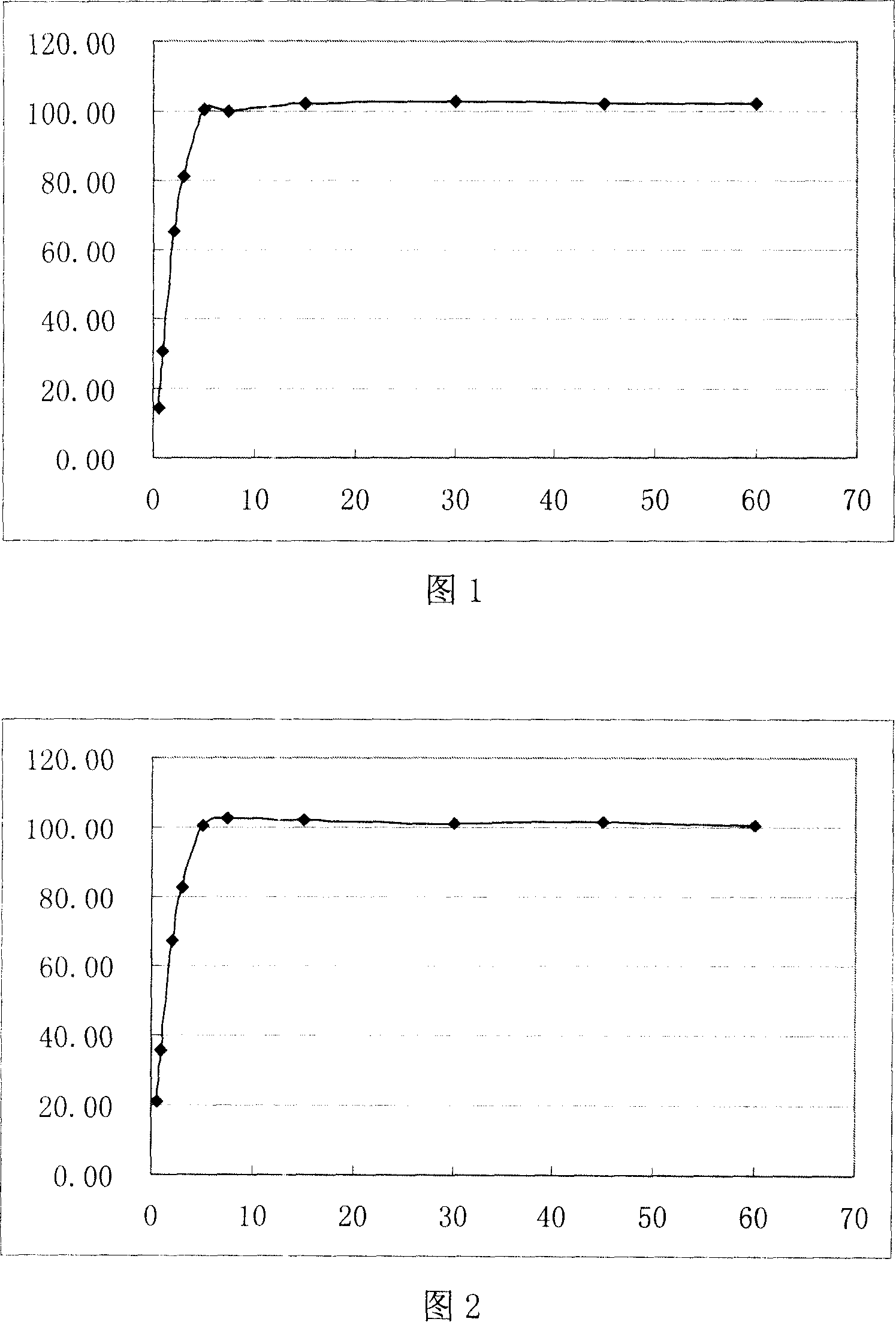
Embodiment 1
[0026] Mirtazapine 15g
[0027] Aspartame 2g
[0028] Menthol 1g
[0029] Orange flavor 4g
[0030] Low-substituted hydroxypropyl cellulose 20g
[0031] Cross-linked polyvinylpyrrolidone 16g
[0032] Sodium carboxymethyl starch 32g
[0033] Microcrystalline Cellulose 80g
[0034] Mannitol 222g
[0035] Micronized silica gel 4g
[0037] 0.2% Peppermint Oil 30% Ethanol Solution Appropriate amount
[0038]
[0039] Makes 1000 pieces
[0040] Preparation Process
[0041] Grind the raw material mirtazapine into fine powder, and pass through an 80-mesh sieve.
[0042] Grind the excipients aspartame, menthol, orange essence, low-substituted hydroxypropyl cellulose, cross-linked polyvinylpyrrolidone, sodium carboxymethyl starch, microcrystalline cellulose, and mannitol into fine powder, and pass through a 80-mesh sieve .
[0043] Mix two-thirds of the total amount of low-substituted hydroxypropyl cel...
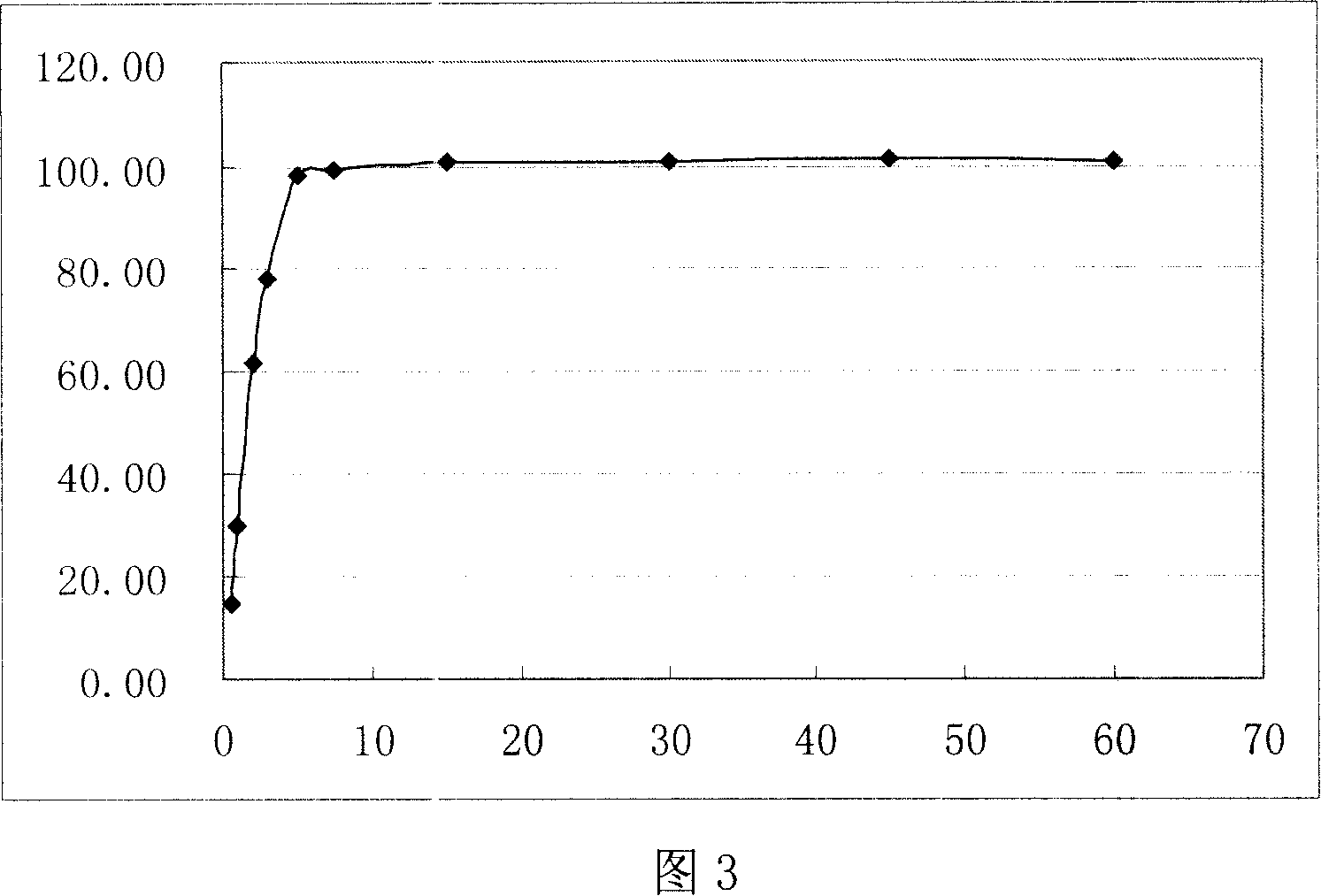
Embodiment 2
[0046] Mirtazapine 45g
[0047] Stevia 10g
[0048] Strawberry essence 4g
[0049] Sodium carboxymethyl starch 50g
[0050] Lactose 145g
[0051] Mannitol 180g
[0052] Micronized silica gel 8g
[0054] Appropriate amount of water
[0055]
[0056] Makes 1000 pieces
[0057] Preparation Process
[0058] Grind the raw material mirtazapine into fine powder, and pass through an 80-mesh sieve.
[0059] The auxiliary materials stevia, strawberry essence, sodium carboxymethyl starch, lactose, and mannitol are respectively ground into fine powder, and passed through a 80-mesh sieve.
[0060] The fine powder of raw and auxiliary materials is fully mixed according to the method of equal increase, passed through a 60-mesh sieve for 2-3 times, and an appropriate amount of water is added to make a soft material, passed through a 20-mesh sieve to granulate, and dried at 55°C±5°C.
[0061] After the dried gra...
Embodiment 3
[0063] Mirtazapine 3g
[0064] 12g powdered sugar
[0065] Lemon essence 5g
[0066] Croscarmellose Sodium 50g
[0067] Microcrystalline Cellulose 100g
[0068] Mannitol 105g
[0069] Micronized silica gel 12g
[0070] Macrogol 6000 6g
[0072] 0.5%PVPk 30 50% ethanol solution appropriate amount
[0073]
[0074] Makes 1000 pieces
[0075] Preparation Process
[0076] Grind the raw material mirtazapine into fine powder, and pass through an 80-mesh sieve.
[0077] The auxiliary materials granulated sugar, croscarmellose sodium, microcrystalline cellulose, and mannitol are respectively ground into fine powder, and passed through a 80-mesh sieve.
[0078] Mix the fine powder of the raw and auxiliary materials according to the method of equal increments, pass through a 60-mesh sieve for 2-3 times, and add 0.5% PVPk 30 A proper amount of 50% ethanol solution is made into a soft material, granulated thro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com