Aripiprazole sustained-release microspheres and preparation method thereof
A technology of aripiprazole and sustained-release microspheres, which is applied in the field of aripiprazole sustained-release microspheres and its preparation, can solve problems such as difficult timely control of adverse drug reactions and complicated preparation techniques, and achieve improved compliance and enhanced Good curative effect and uniform effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
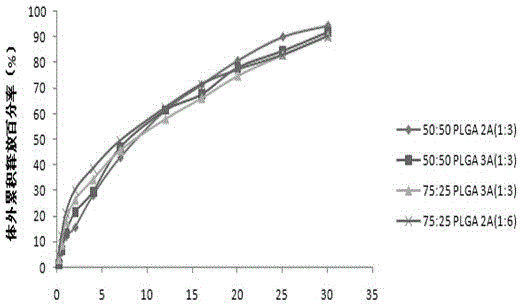
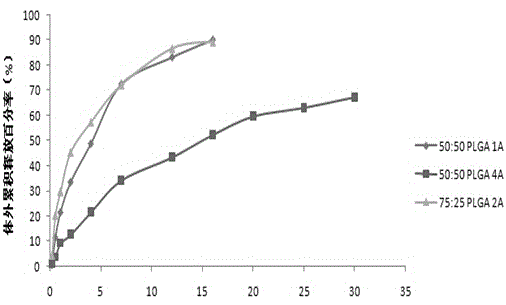
[0027] Weigh 220.51mg of PLGA (50 / 50-2A, molecular weight 25000 Daltons), 73.39mg of aripiprazole, add 4mL of dichloromethane and acetonitrile (3:1) mixed organic solvent, stir to dissolve, and form an oil phase (O ), quickly drop the oil phase into 100mL of PVA water phase (W) containing 1.5%, and emulsify at 10000rpm for 90s at high speed to form an O / W emulsion, and then dilute the water phase with purified water to double the obtained The O / W type emulsion was placed on a magnetic stirrer at 800 rpm and stirred overnight to volatilize the organic solvent to solidify the microspheres. The microspheres were collected by centrifugation, washed with purified water, and then vacuum freeze-dried to obtain aripiprazole microspheres. The drug loading was 20.63%, and the encapsulation efficiency was 82.52%.
Embodiment 2~7
[0030]
[0031] The microsphere preparation step of embodiment 2~7 is the same as embodiment 1
Embodiment 8~14
[0034] The prescription of table 2 embodiment 8~14 slow-release microspheres
[0035]
[0036] The microsphere preparation step of embodiment 8~14 is the same as embodiment 1
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com