Solid oral medicine composition containing aripiprazole microcrystal
A technology of aripiprazole and its composition, which is applied in the field of solid oral pharmaceutical compositions, can solve the problems of low bioavailability and achieve the effects of high bioavailability, improved bioavailability, and uniform and stable content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
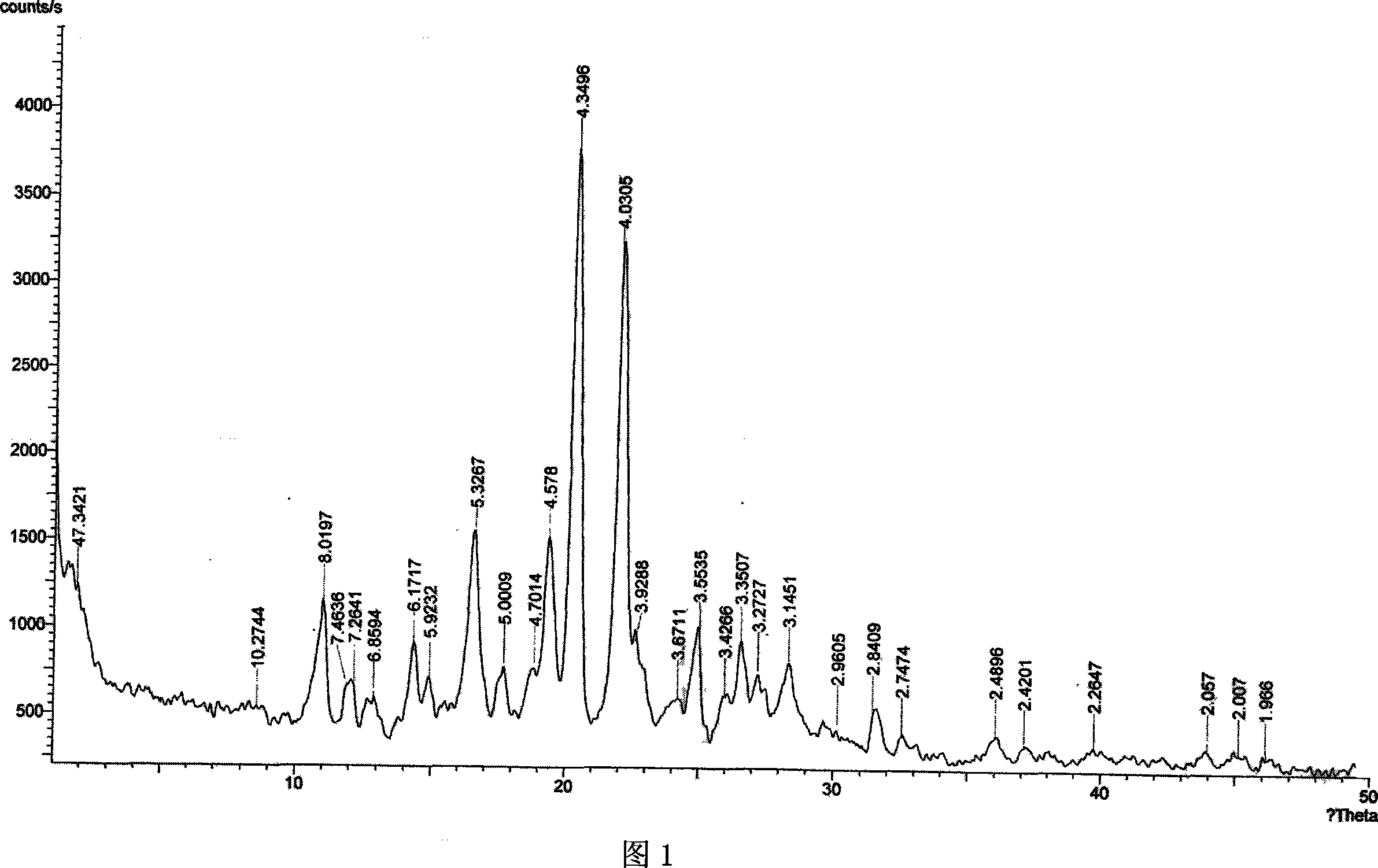
[0033] Preparation of Type I Microcrystals of Aripiprazole
[0034] Add 20g of crude aripiprazole and 240ml of ethanol into a three-necked reaction flask with a reflux condenser, heat to reflux under stirring, stop heating after the aripiprazole is completely dissolved, adjust the line speed to 500 m / min, and add 1 37ml of low-temperature water at ℃, cooled rapidly with ice-water mixture for 30min, suction filtered, washed, and the obtained crystals were dried in a desiccator at 80℃ for 10 hours under reduced pressure to obtain 19.2g of powdery aripiprazole crystals. By using water as a dispersant, it is measured with a Mastersizer 2000 laser particle size analyzer, and its volume average particle diameter is 26.456um.
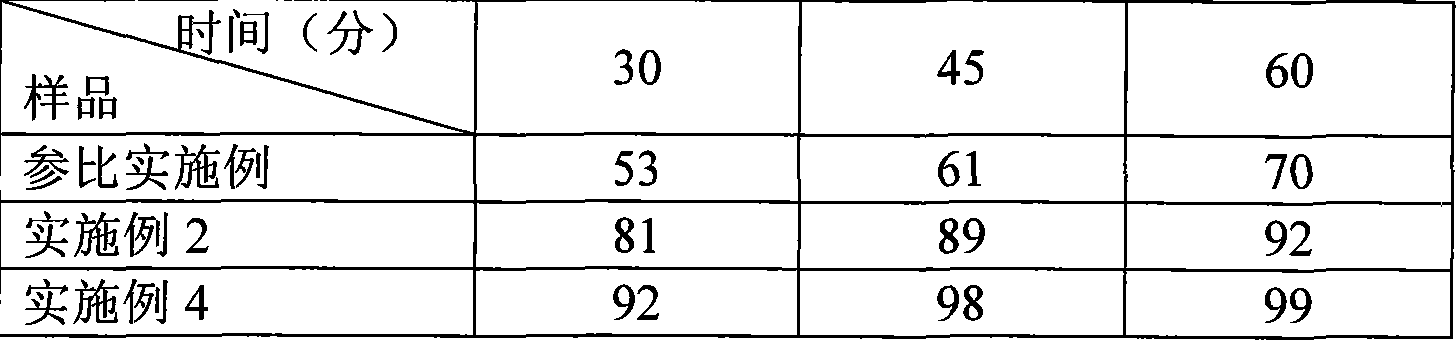
Embodiment 2
[0036] Aripiprazole type I microchips (except type I microcrystals, the prescription is the same as the reference example, and the preparation method of microcrystals is shown in the examples)
[0037] prescription:
[0038] Aripiprazole (type I microcrystalline) 15g
[0039] Lactose 57g
[0040] Starch 10g
[0041] Microcrystalline Cellulose 10g
[0042] Hypromellose 2g
[0043] Magnesium stearate 0.9g
[0044] 94.9g 1000 pieces
[0045] Preparation process: with reference example.
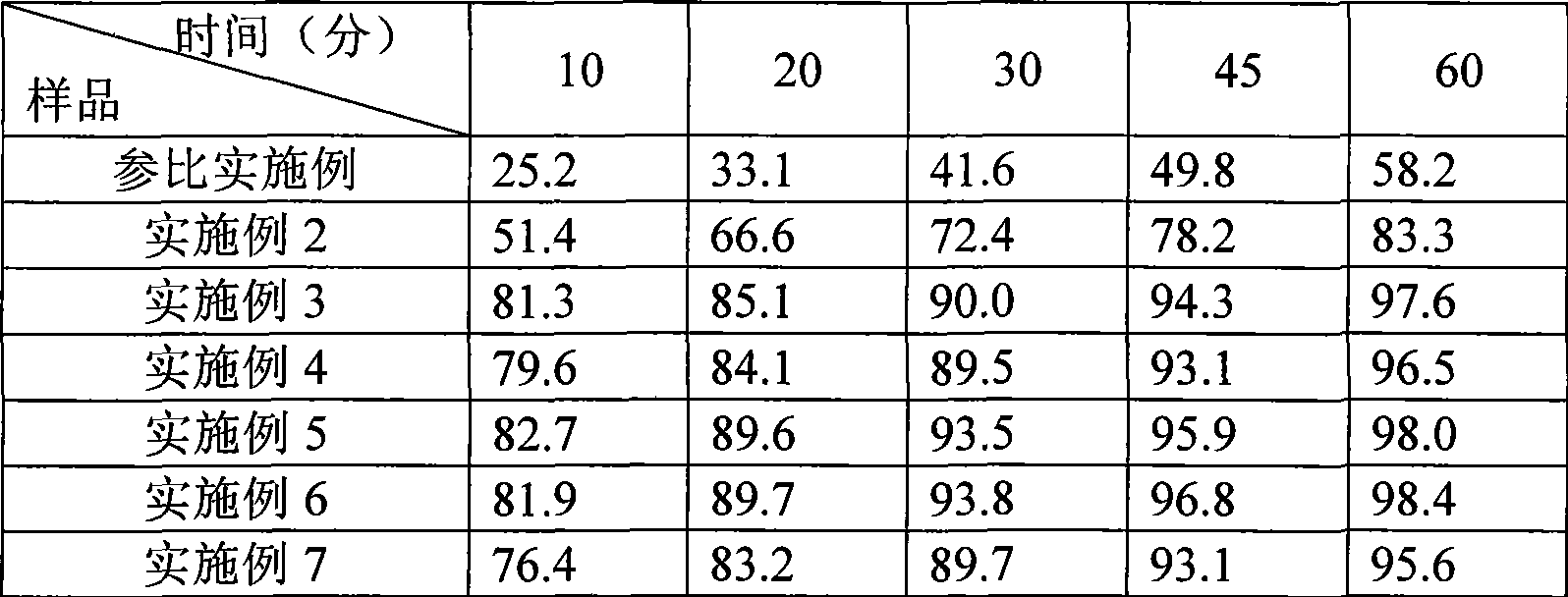
Embodiment 3
[0047] Aripiprazole type I microchips
[0048] prescription
[0049] Aripiprazole (type I microcrystalline) 5g
[0050] Lactose 75g
[0051] Microcrystalline Cellulose 11g
[0052] Sodium starch glycolate 2.8g
[0053]Hypromellose 2.2g
[0054] Sodium starch glycolate (additional) 3.2g
[0055] Magnesium stearate 0.8g
[0056] 100g 1000 pieces
[0057] Preparation: Mix aripiprazole type I microcrystalline, hydroxymethyl starch sodium, microcrystalline cellulose, starch and lactose evenly, add hypromellose alcohol solution, mix, granulate, dry, sieve, and then add Sodium carboxymethyl starch, mix well, then add magnesium stearate, mix well, press into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Volume average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com