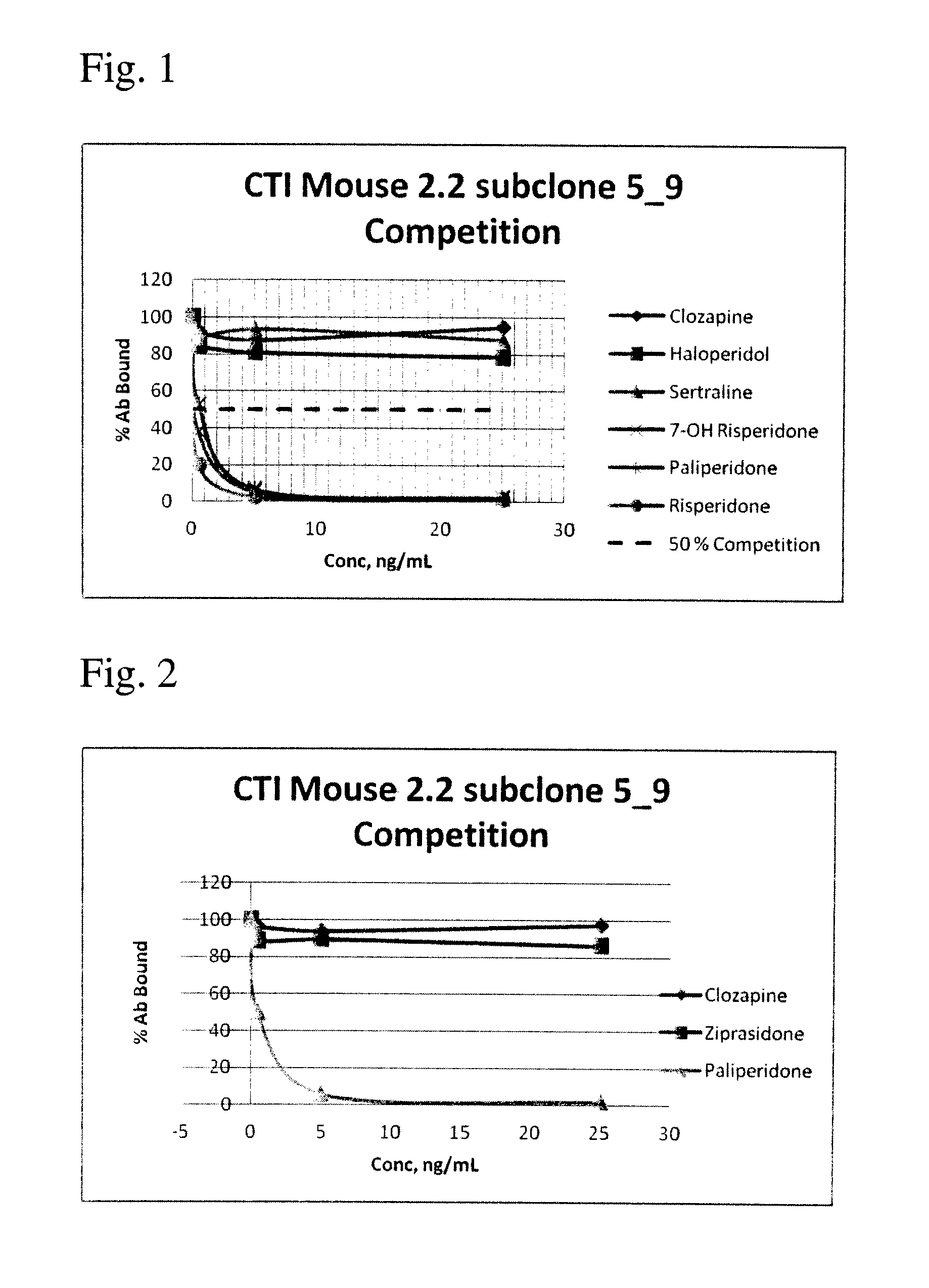
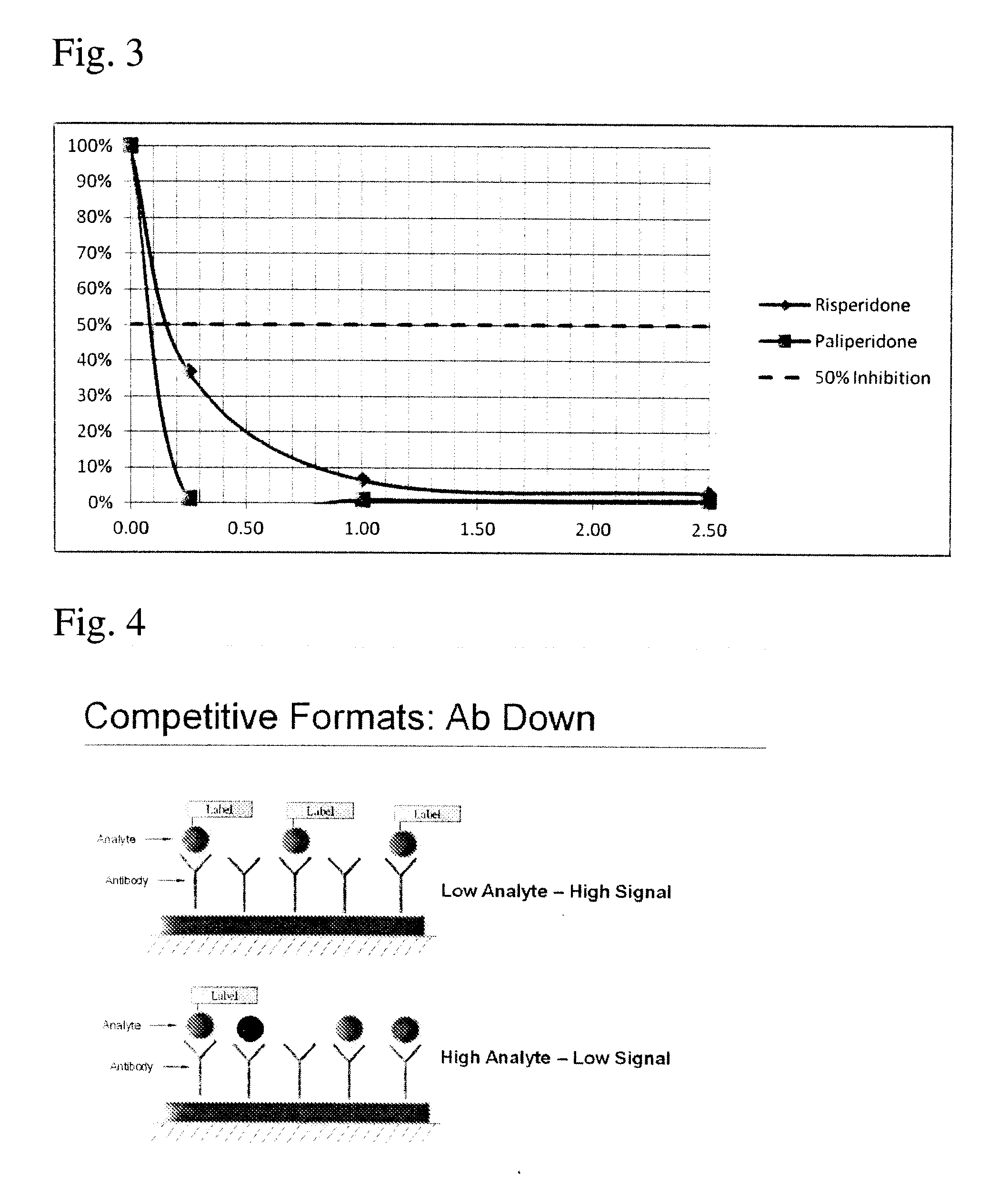
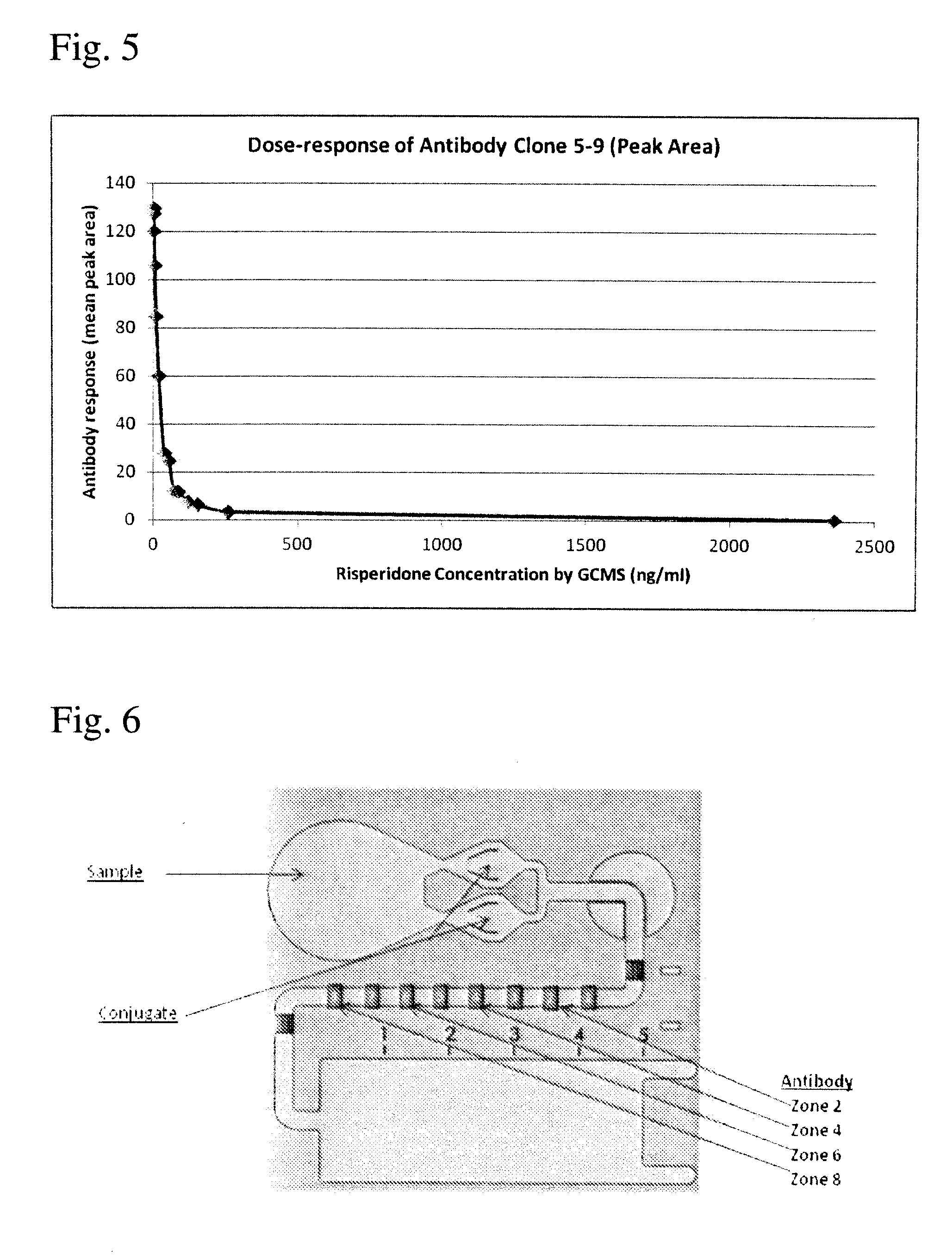
Antibodies to Paliperidone Haptens and Use Thereof
a technology of anti-haptens and paliperidone, which is applied in the field of immunoassays, can solve the problems of increased mortality, poor adherence to medication, and relapse of daily administered oral medications, and achieves the effects of improving survival rate, hospital admission and suicide, and improving drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
4-((3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-2-methyl-4-oxo-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-9-yl)oxy)-4-oxobutanoic acid
[0150]
[0151]Paliperidone (10 g, 23.45 mmol) was dissolved in pyridine (100 mL) together with N,N-dimethyl-4-pyridinamine (0.5 g; 4.09 mmol) and succinic anhydride (18.3 g; 182.86 mmol). The mixture was stirred under an argon atmosphere for 4 h at 60° C. and for 48 h at room temperature. The reaction mixture was evaporated and dissolved in dichloromethane / methanol (100 mL / 10 mL) and water (100 mL). The aqueous phase was extracted five times with dichloromethane / methanol (100 mL / 10 mL). The organic layers were combined, dried over Na2SO4 and evaporated under vacuum. The residue was purified by silica gel chromatography (gradient elution with chloroform / ethanol 80 / 20 to 70 / 30). The product was dissolved in isopropanol (50 mL) and the resulting precipitate was filtered and washed with isopropanol (10 mL). The precipitate was dried in va...
example 2
Step A 3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-2-methyl-4-oxo-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-9-yl 3-pivalamidopropanoate
[0152]
[0153]A solution of Paliperidone (1.0 g, 2.34 mmol) in dichloromethane (20 mL) was treated with Boc-b-Ala-OH (443.65 mg, 2.34 mmol), dicyclohexylcarbodiimide (483.79 mg, 2.34 mmol) and N,N-dimethyl-4-pyridinamine (14.32 mg, 0.117 mmol). The reaction was stirred at room temperature under argon atmosphere. After 18 h, the reaction mixture was poured into an aqueous saturated sodium bicarbonate solution (20 mL) and the aqueous layer extracted with dichloromethane (three times 20 mL). The combined organic layers were dried over MgSO4, filtered and concentrated. The residue was purified by silica gel chromatography (elution with dichloromethane / methanol (95 / 5) to give the title compound. ESI-MS (M+1) 598.
Step B 3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-2-methyl-4-oxo-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin...
example 3
Step A N-(3-((3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-2-methyl-4-oxo-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-9-yl)oxy)propyl)pivalamide
[0156]
[0157]A solution of paliperidone (746.26 mg, 1.75 mmol) in THF (15 mL) was treated with 3-(Boc-amino)propyl bromide (500 mg, 2.10 mmol), 18-Crown-6 (231.25 mg, 0.874 mmol) and sodium hydride (60 w / w %, 139.97 mg, 3.5 mmol). The mixture was stirred at room temperature for 6 h. The solvent was removed under vacuum, dissolved in dichloromethane / water (50 mL / 50 mL) and the aqueous layer extracted with dichloromethane (three times 50 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated to give the title compound, which was used without further purification in the next step.
Step B 9-(3-aminopropoxy)-3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one
[0158]
[0159]A solution of N-(3-((3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com