Patents
Literature
38 results about "Poor adherence" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
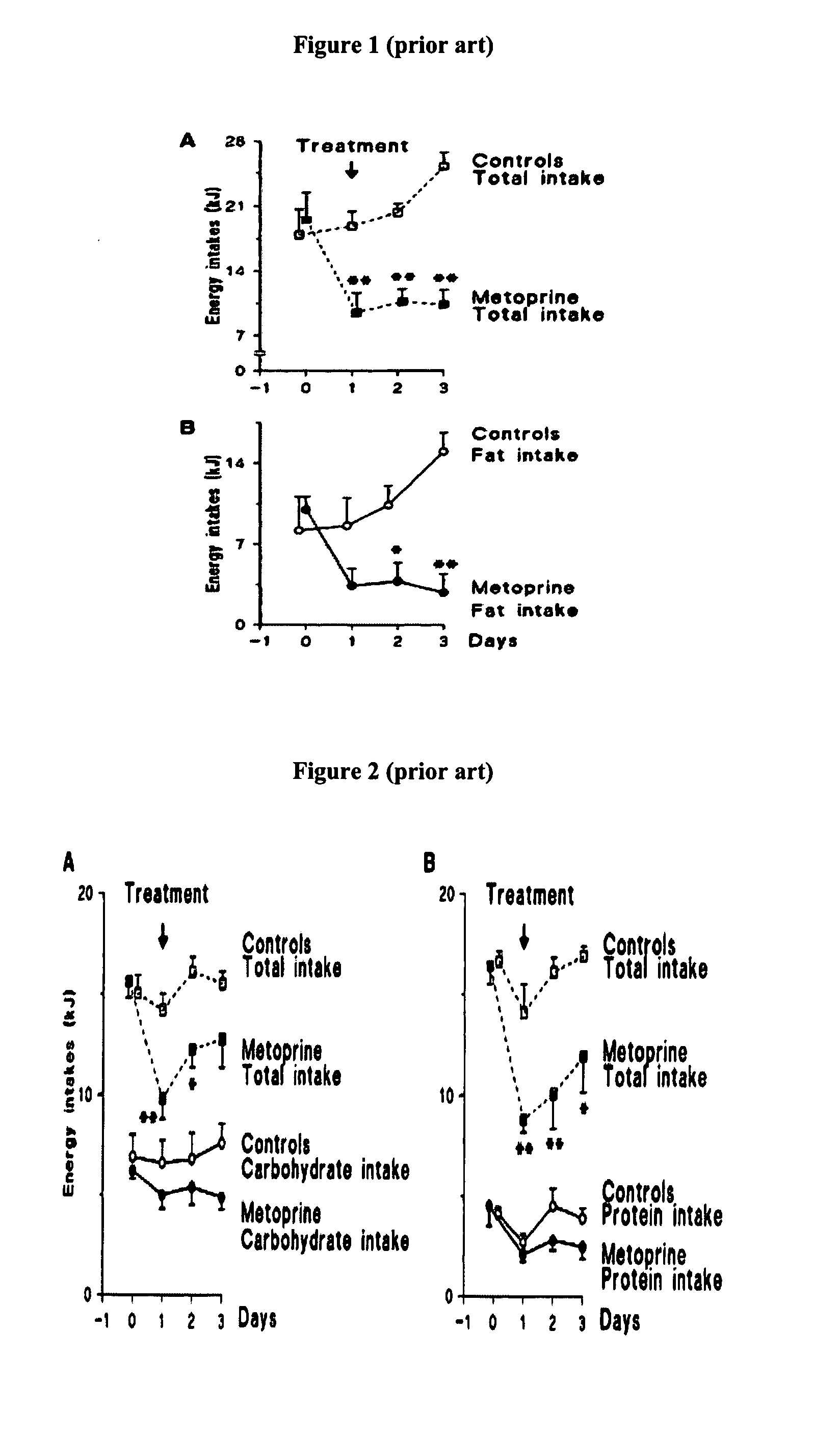
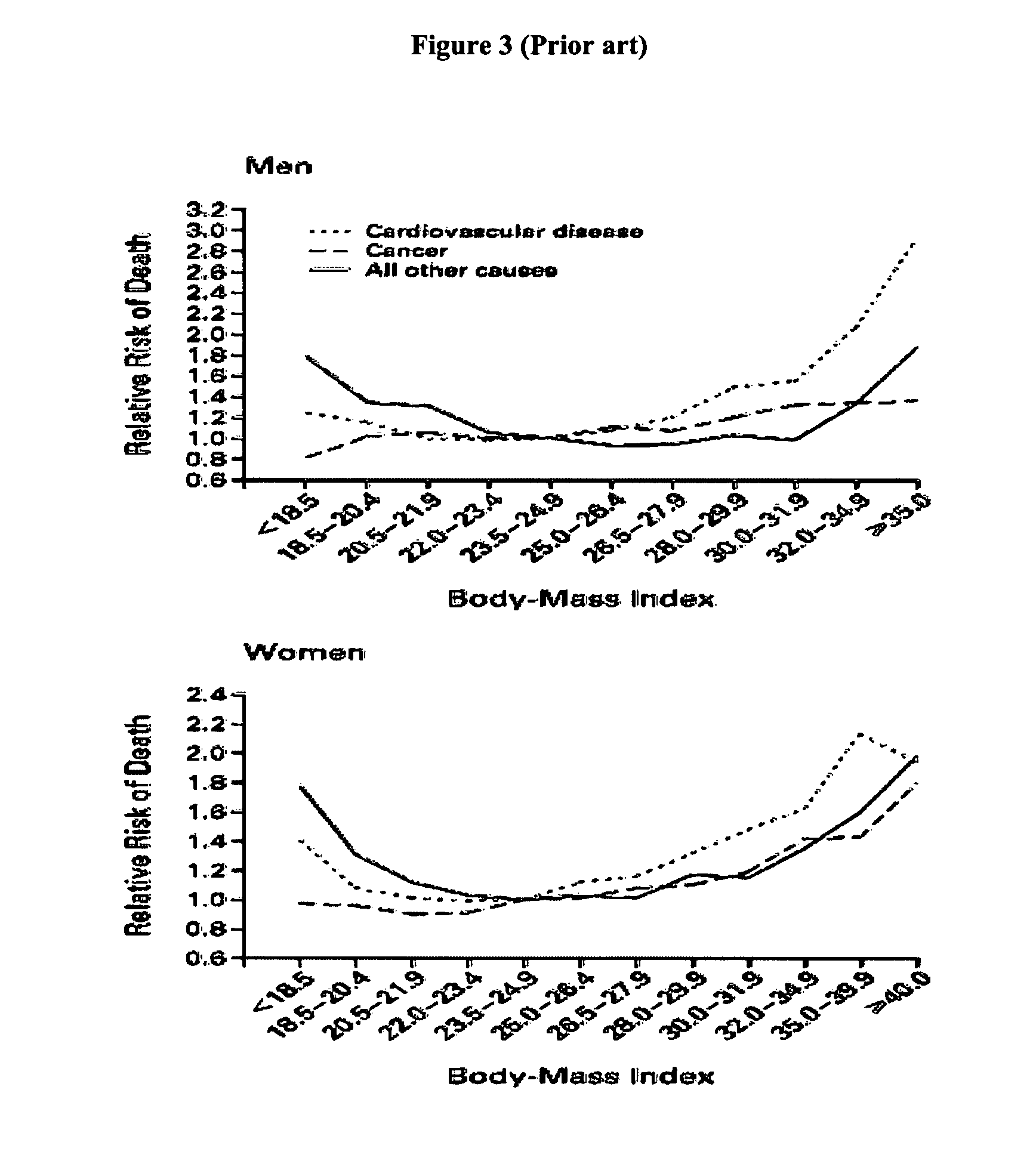
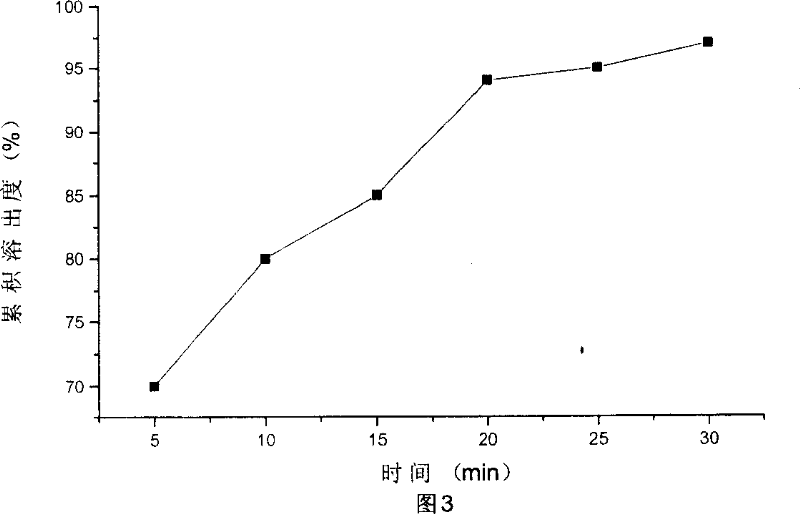
Forgetfulness is the most common patient factor leading to poor adherence, while personal or emotional issues can also affect adherence. 16. Providers may unintentionally influence adherence due to limited familiarity with drug costs and/or limited knowledge of insurance coverage and formularies.
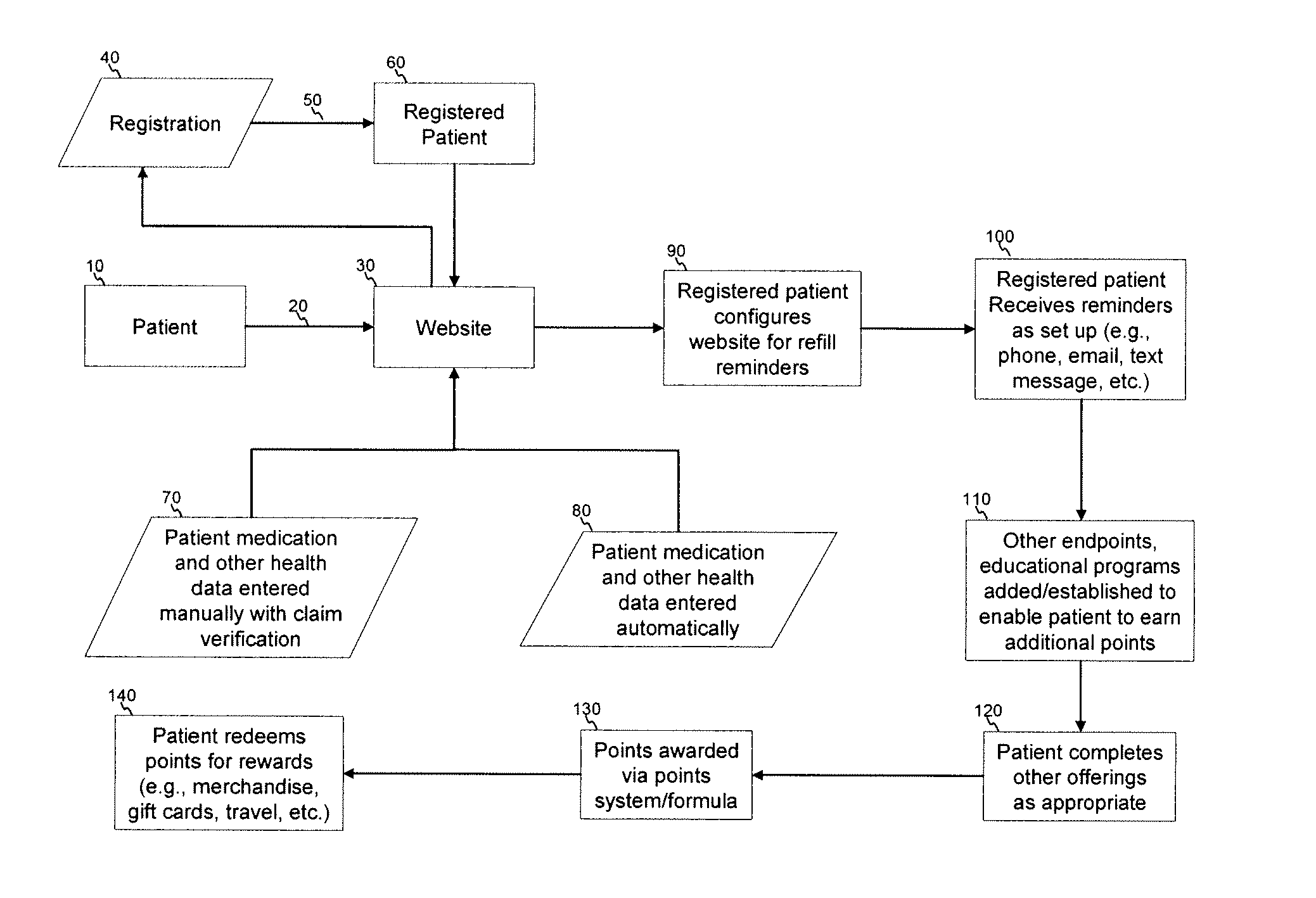
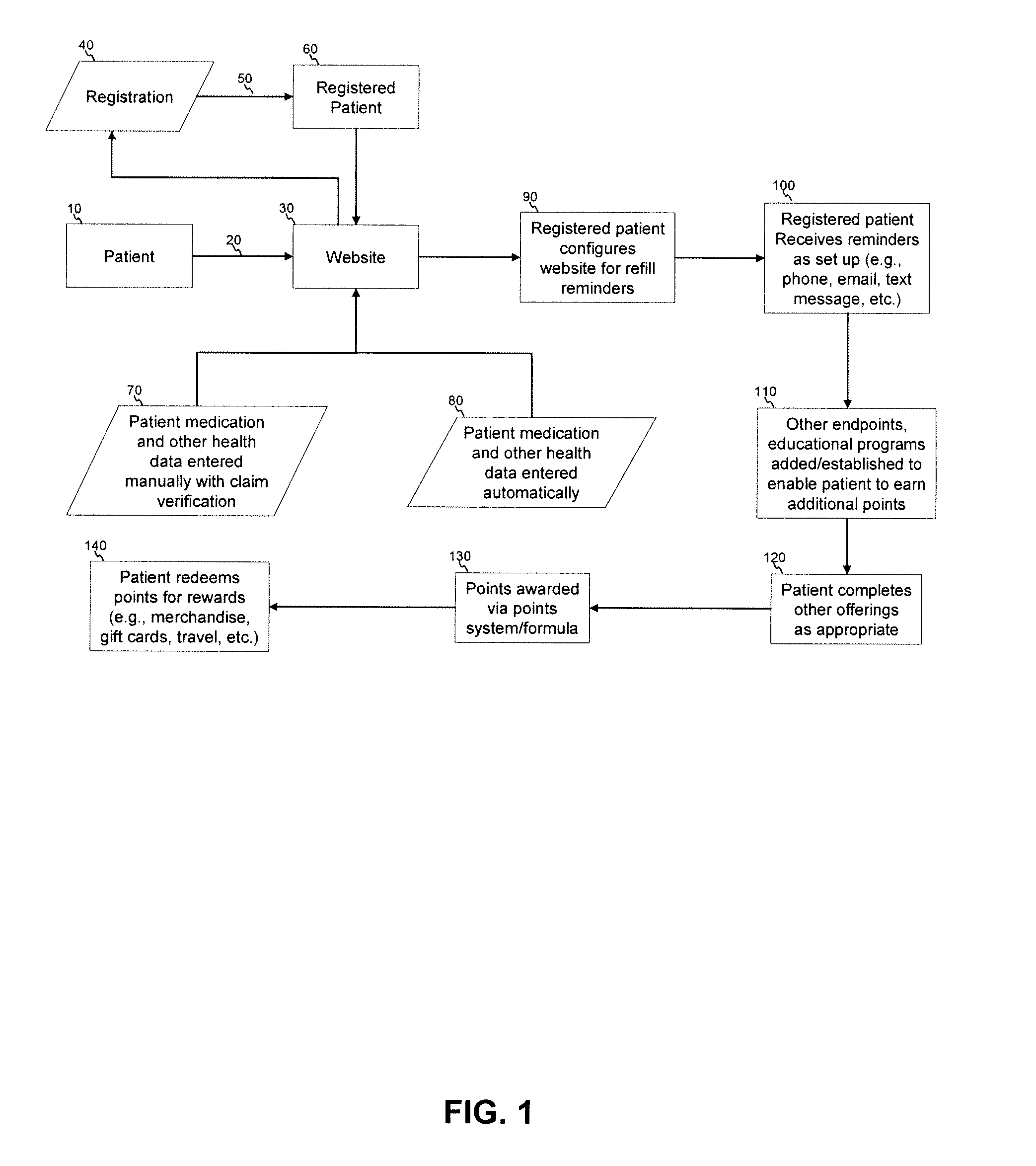
Apparatus and method for prediction and management of participant compliance in clinical research
InactiveUS7415447B2Reduce testing costsImprove statistics performanceDigital computer detailsForecastingNon complianceClinical trial
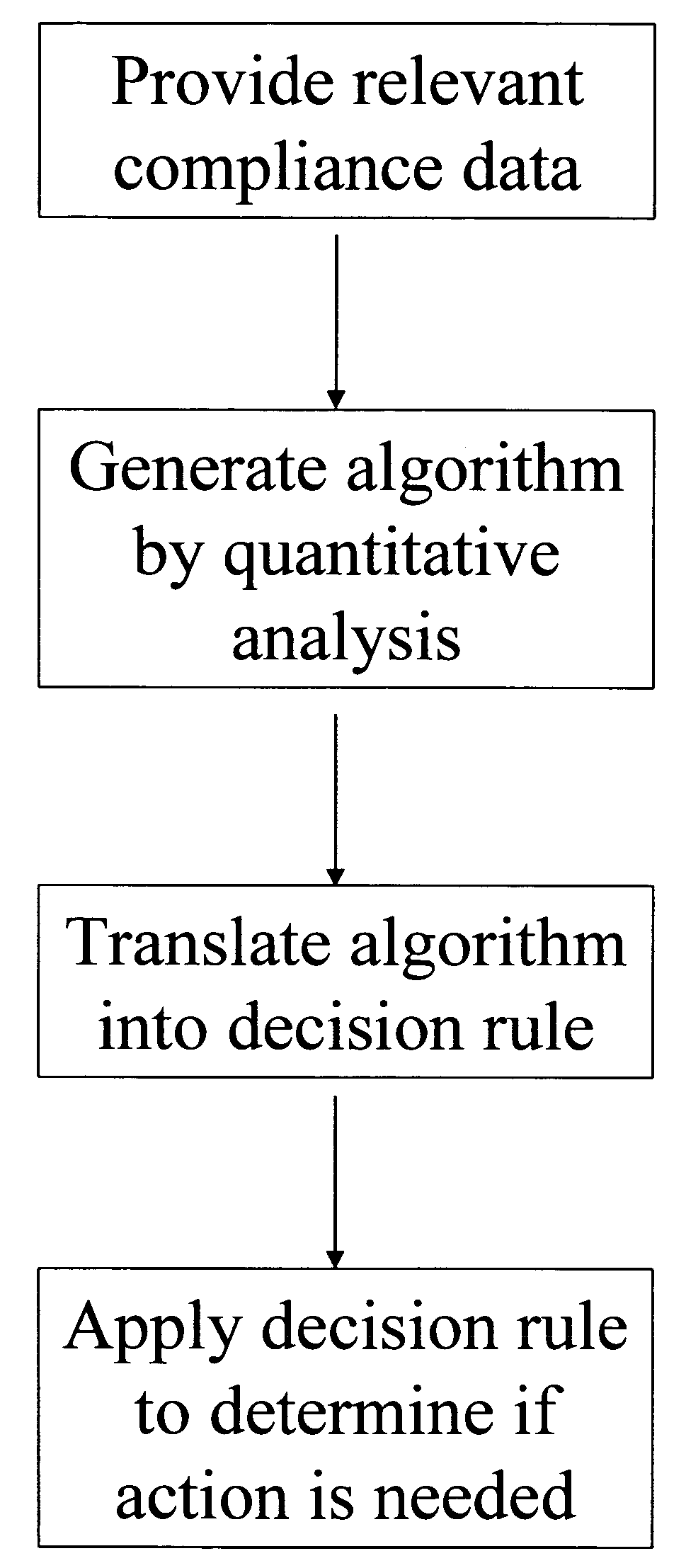
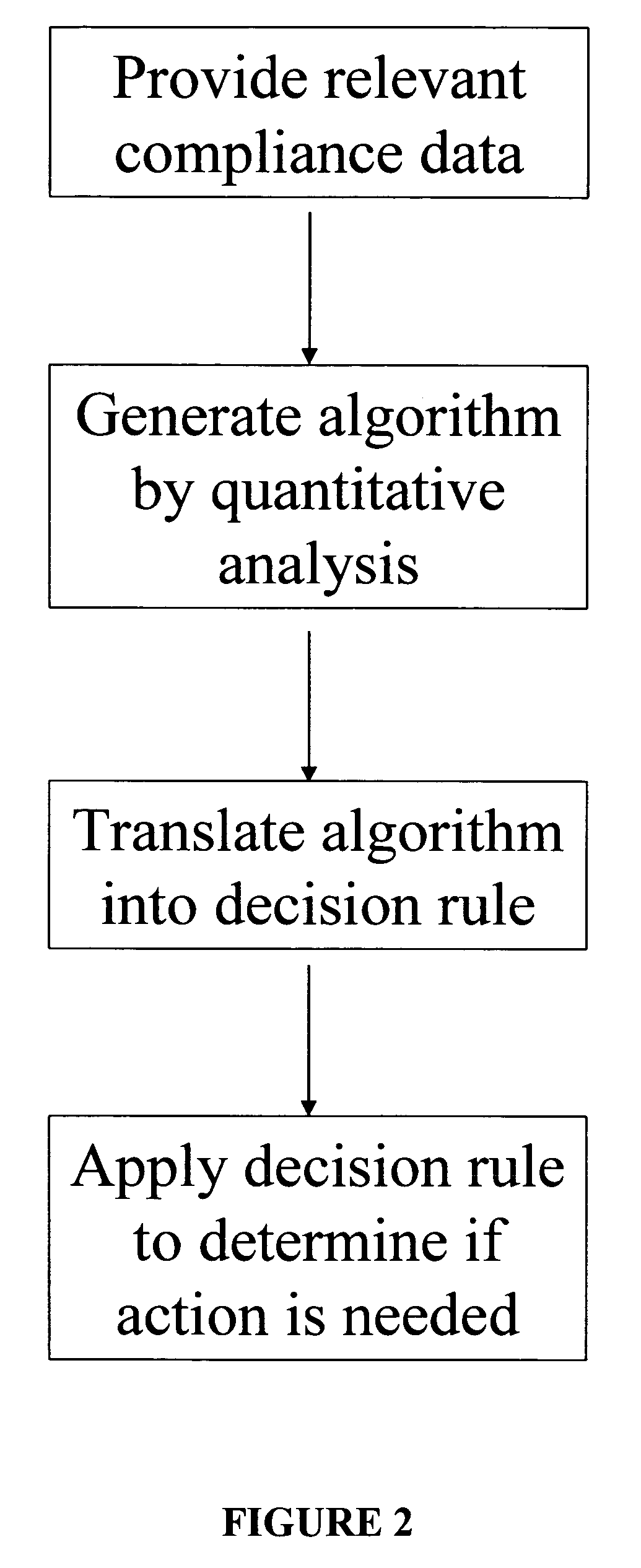
A system for developing and implementing empirically derived algorithms to generate decision rules to determine participant noncompliance and fraud with research protocols in clinical trials allows for the identification of complex patterns of variables that detect or predict participant noncompliance and fraud with research protocol, including performance and enrollment goals, in the clinical trial. The data may be used to overall predict the performance of any participant in a clinical trial, allowing selection of participants that tend to produce useful, high-quality results. The present invention can also be used to monitor participant compliance with the research protocol and goals to determine preferred actions to be performed. Optionally, the invention may provide a spectrum of noncompliance, from minor noncompliance needing only corrective feedback, to significant noncompliance requiring participant removal from the clinical trial or from future clinical trials. The algorithms and decision rules can also be domain-specific, such as detecting non-compliance or fraud among subjects in a cardiovascular drug trial, or demographically specific, such as taking into account gender, age or location, which provides for algorithms and decision rules to be optimized for the specific sample of participants being studied.
Owner:ERESTECH
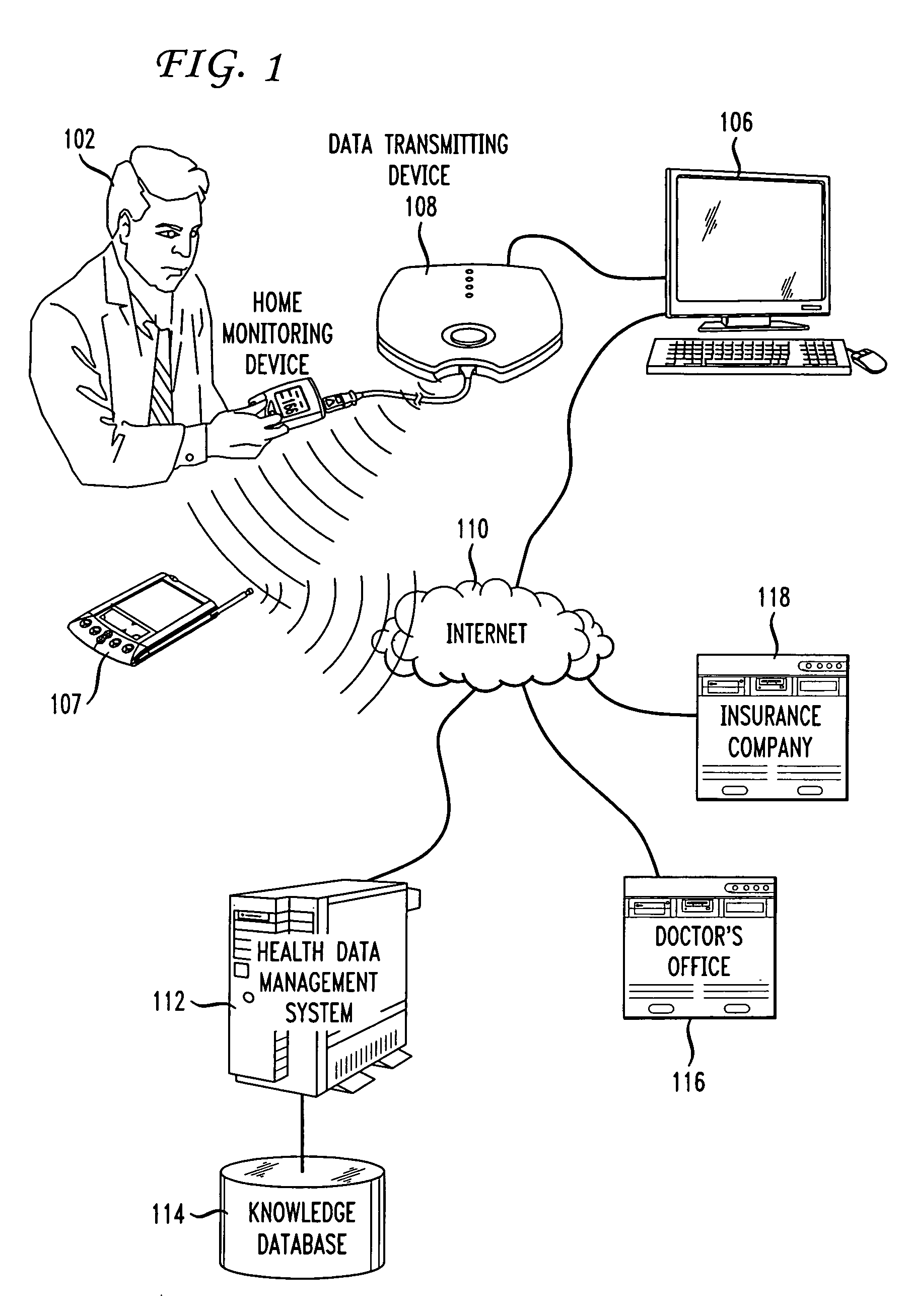
Methods and systems for monitoring and enhancing patient compliance with a health treatment program
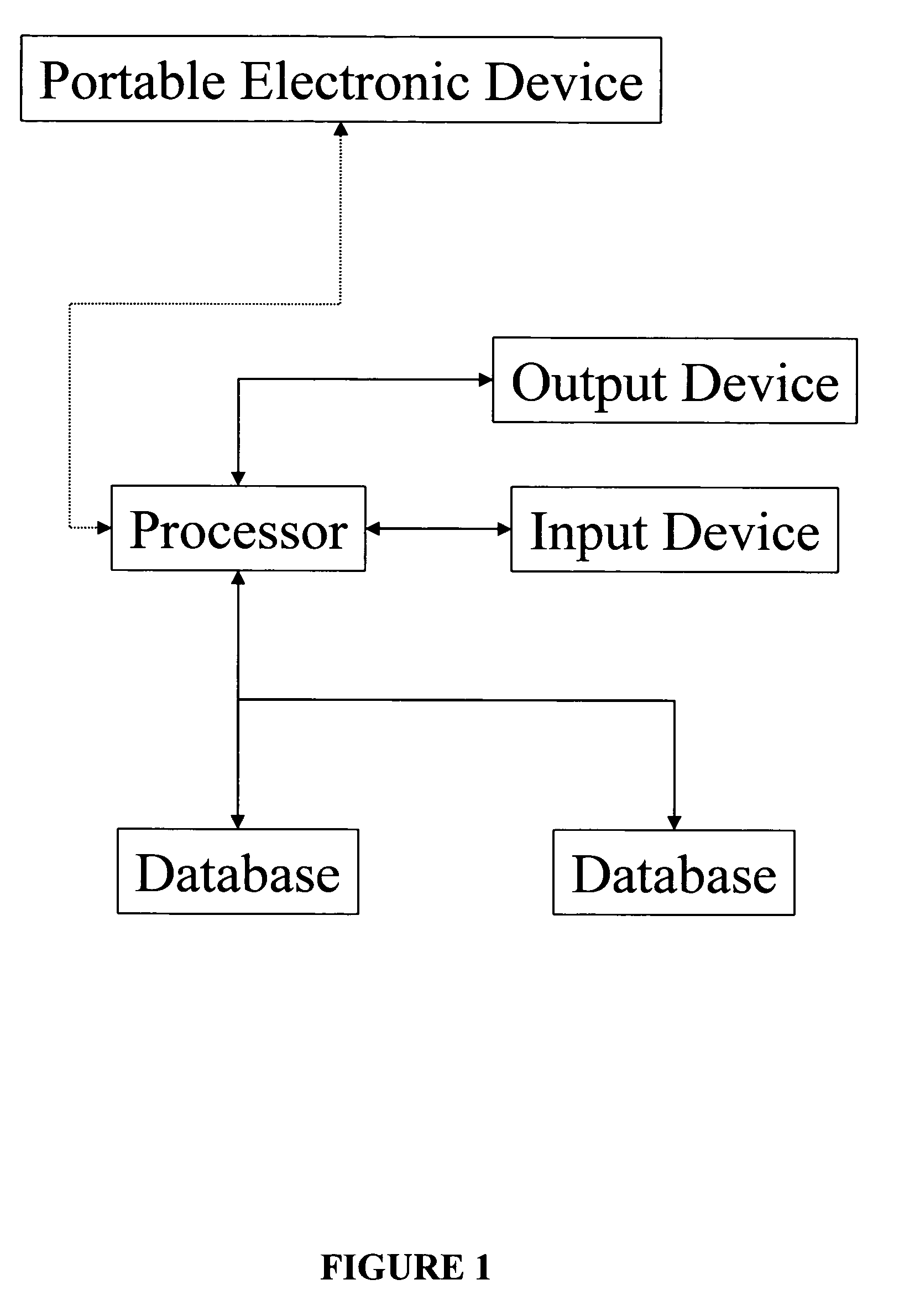
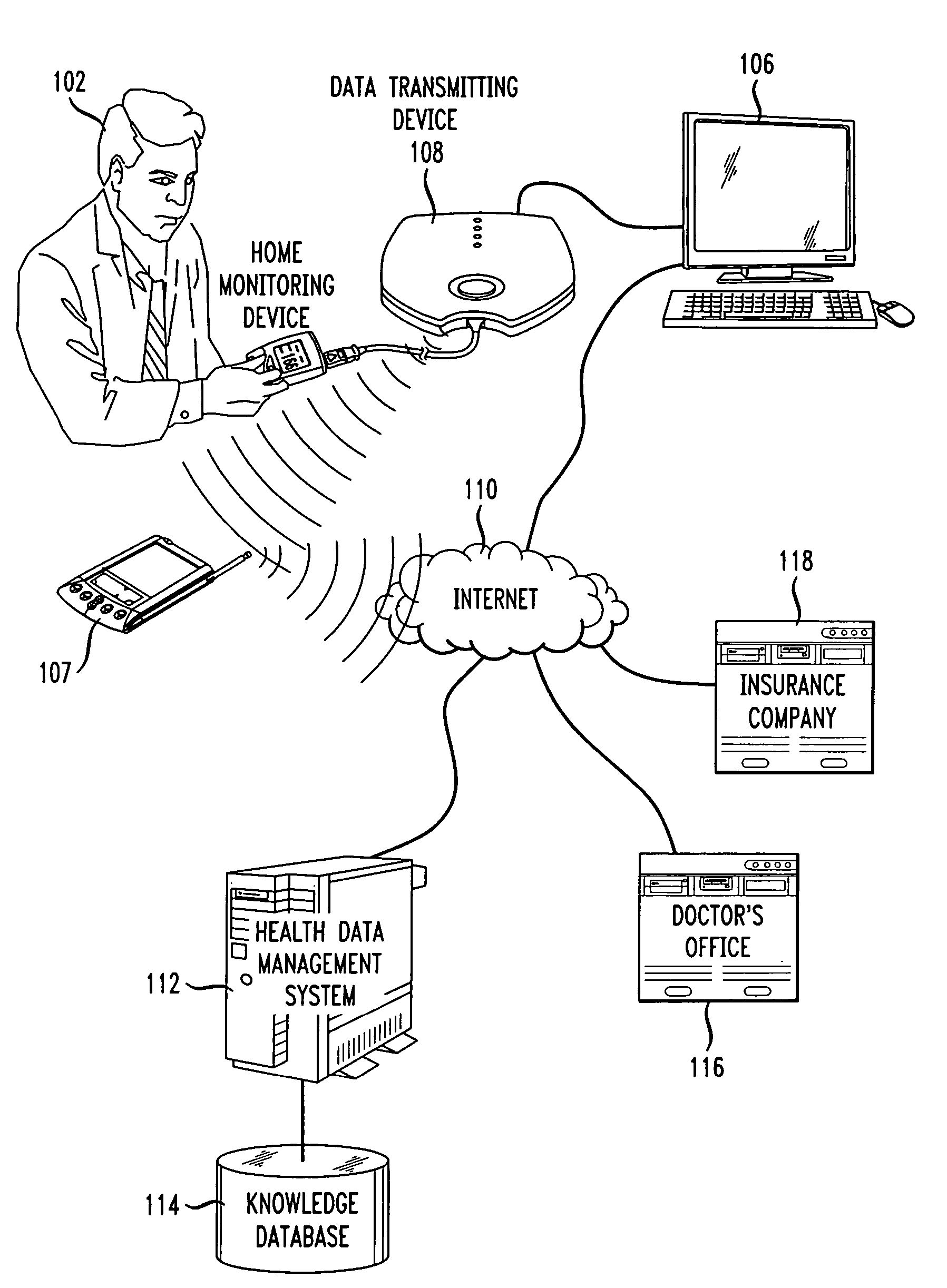
Methods and systems for enabling and supporting patient compliance with a health treatment program or with health-related instructions from a caregiver are described. An interactive feedback loop is implemented that enhances and improves on present methods of monitoring and supporting patient compliance with treatment programs, particularly for patients with chronic conditions. A patient enters various types of data (e.g., biometric readings, diary entries, responses to surveys and health assessments, etc.) to a health data management system that includes a patient compliance monitoring system. The patient is encouraged to enter as much data as is practical and as frequently as possible. The feedback loop of the present invention provides compliance messages to the patient in a timely manner that are responsive to the data entered by the patient. The messages are customized, supportive, and timely. The system can also implement a reward scheme in which patients who go beyond their prescribed treatment program or consistently stay within a compliance range obtain direct financial benefits or rewards. The system also allows patients to sends feedback on the messages he or she receives from the system. This feedback can be used to measure the effectiveness of a treatment program or of the monitoring system itself. Healthcare professionals play an active role in tailoring and “signing off” the compliance-related messages that the patient receives.
Owner:IMETRIKUS
Method for monitoring hand hygiene compliance
While clean, disinfected hands are widely recognized as a principle means for controlling the spread of infections, disease, and similar health problems to both self and others, the prompt and repeated application of such materials as soap and water or waterless gels is necessary to maintain sanitary hands. So well recognized is this fact that many professional and governmental guidelines backed by regulations have been drawn to enforce compliance. This invention relates to compliance monitoring of hand sanitizing fluids and gels used to reduce the hand-borne transmission of pathogens. Disclosed is a simple, low-cost method of which a key aspect is the employment of a wristband dispenser with plural individual applications of hand sanitizing fluid in a convenient dispensing form. Inclusion of a use activated timing device provides a means of monitoring an individual's elapsed time since last use.
Owner:HARPER JUDITH LEE +1
Preempt Muscle Map Screen
InactiveUS20120165703A1Easily and accurately measureMonitor program adherence and effectivenessPhysical therapies and activitiesHealth-index calculationMuscle strengthMedicine
Some embodiments of the invention provide a software generated series of tests or screens to determine skeletal muscle balance as related to muscle strength and muscle flexibility. In some embodiments, a color coded muscle map illustrating the findings of the screens, is generated. The findings of the screens compile an exercise program, specifically tailored to the individual's general health and fitness state, as well as to the specific shortcomings identified in the screen. The invention also provides, in some embodiments, an exercise program adherence tracking calendar which monitors both completed exercises and exercise effectiveness as measured by changes in screen results.
Owner:BOTTUM PAUL WILLIAM
Oral respiration interface and a digital container
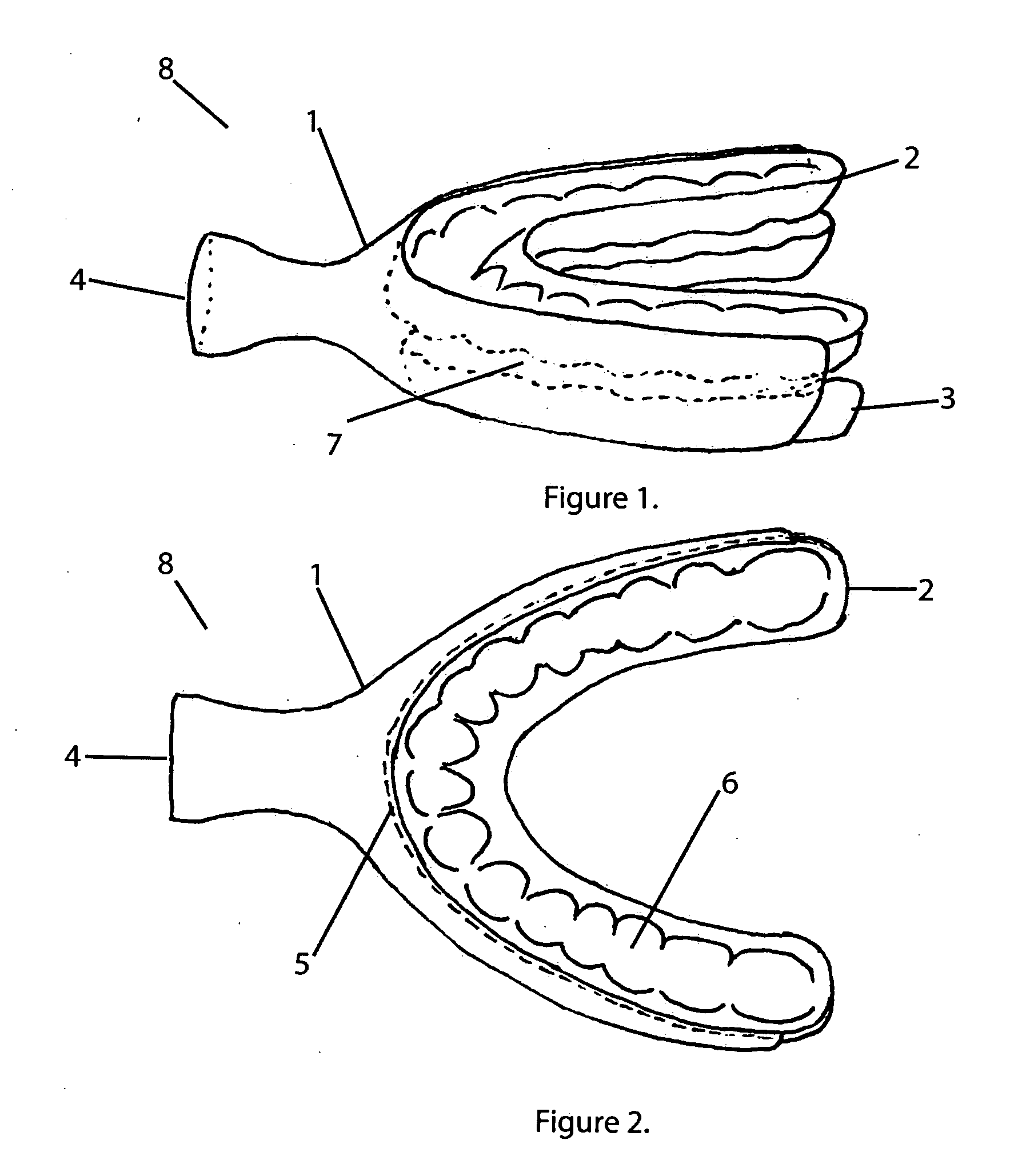
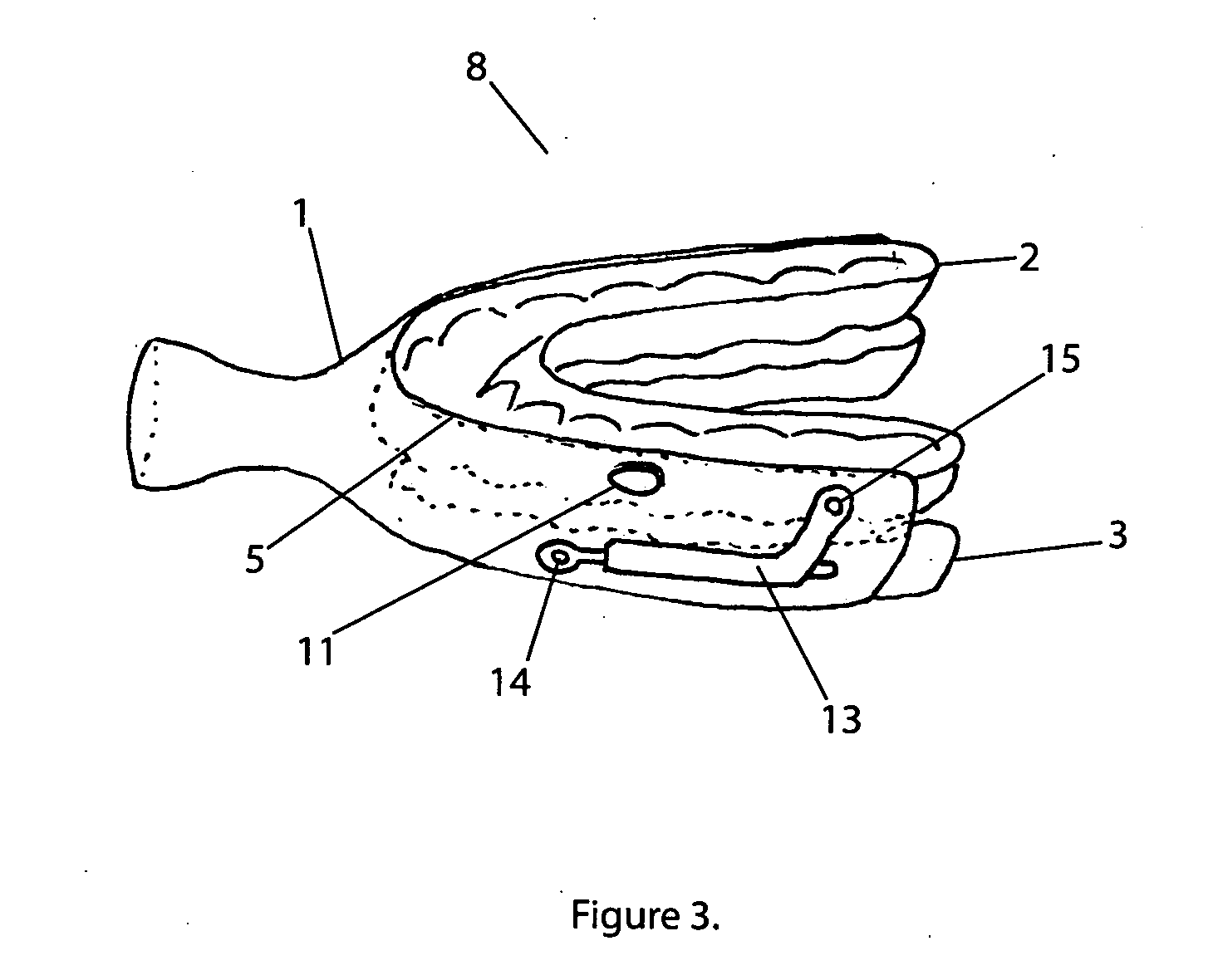
InactiveUS20100311003A1Decreased air pressure settingReduce airway resistanceOthrodonticsSnoring preventionOral appliancePoor adherence
The invention relates to oral appliances configured to maintain users upper airway unobstructed, facilitating improved breathing and elimination of snoring and obstructive sleep apnea. This invention covers mechanical and pneumatic means of maintaining users airway open. A smart container for appliances is also disclosed to be used in conjunction with any oral appliance as a system for wirelessly recording patient biofeedback, treatment compliance and live monitoring of the users medical condition.
Owner:KOZLOV ALEKSEY YURIY
Systems for and Methods of use of Therapeutic Nutrition for the Management of Age-Associated and Age-Specific Health Conditions of the Elderly
InactiveUS20090203606A1Effective therapeutic specific nutritional supportEffective carrierBiocidePeptide/protein ingredientsRegimenNutritional deficiency
Nutritional compositions which provide for improved taste profiles for the elderly while simultaneously providing nutrition specific to assist in the management of nutritional deficiencies that lead to age specific and age associated health conditions in the elderly. There are also provided products using these compositions and incorporating packaging design, volume delivery, and sensory attributes to improve nutritional regimen compliance in the elderly.
Owner:ENERGY LIGHT
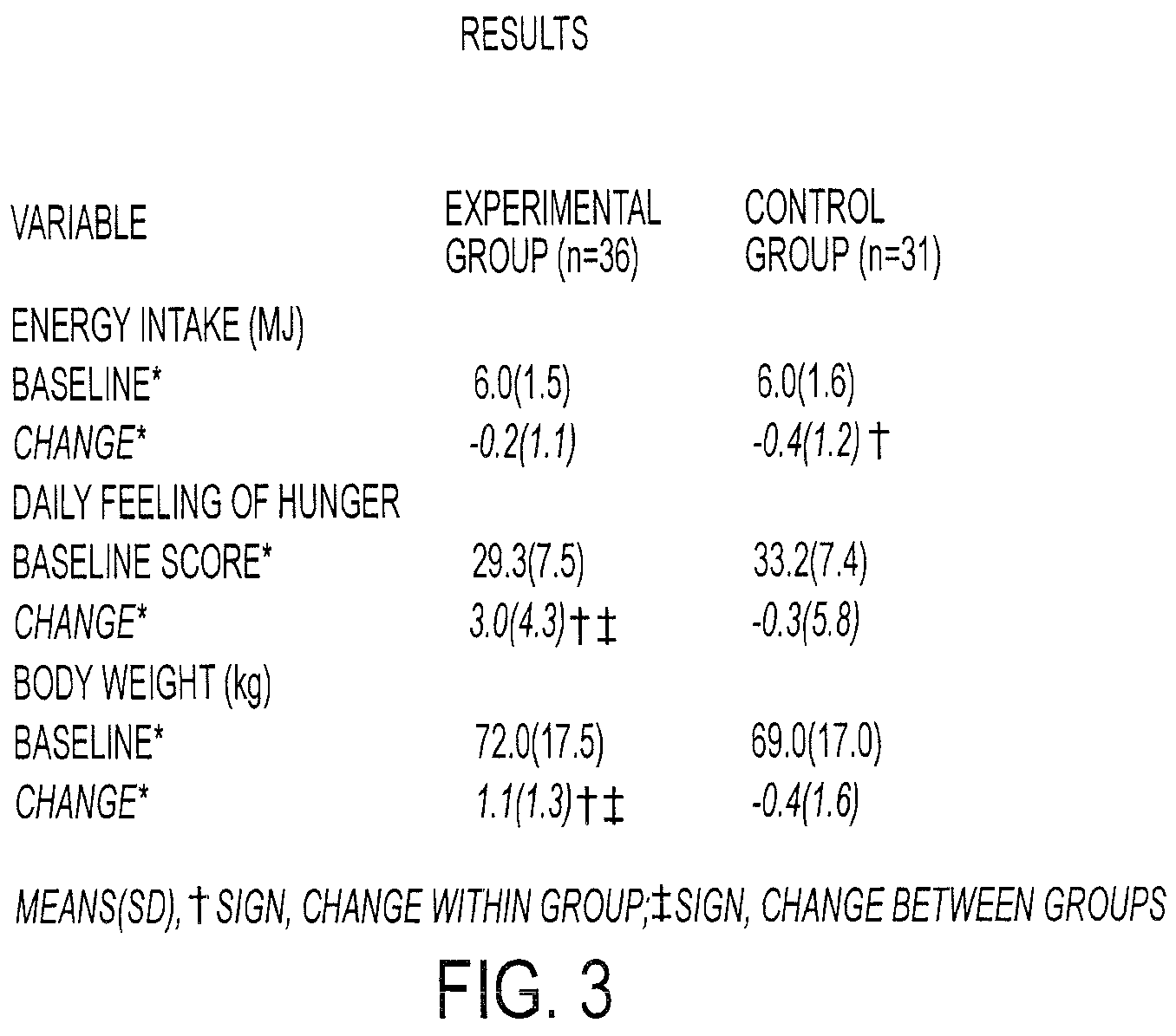
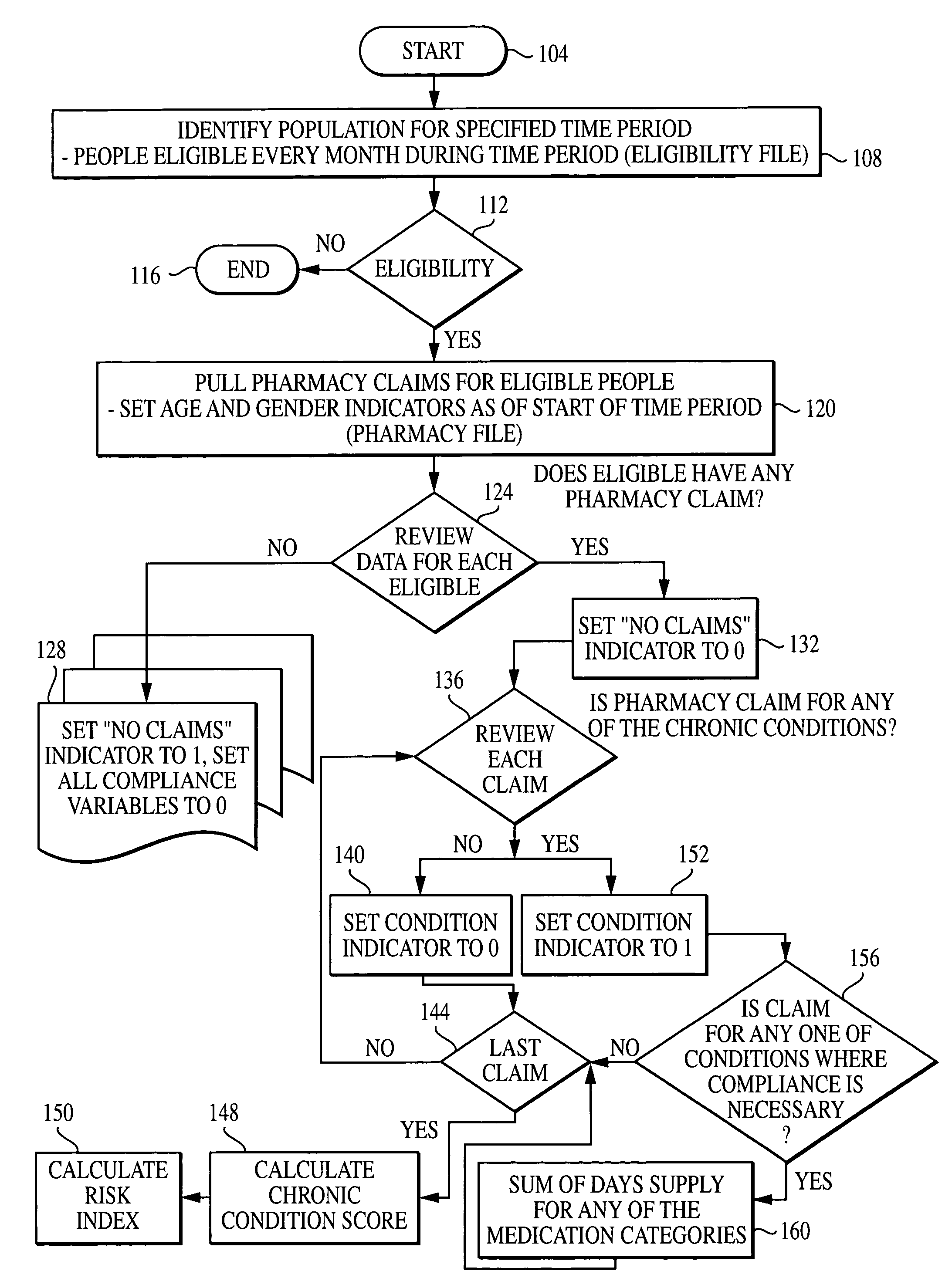
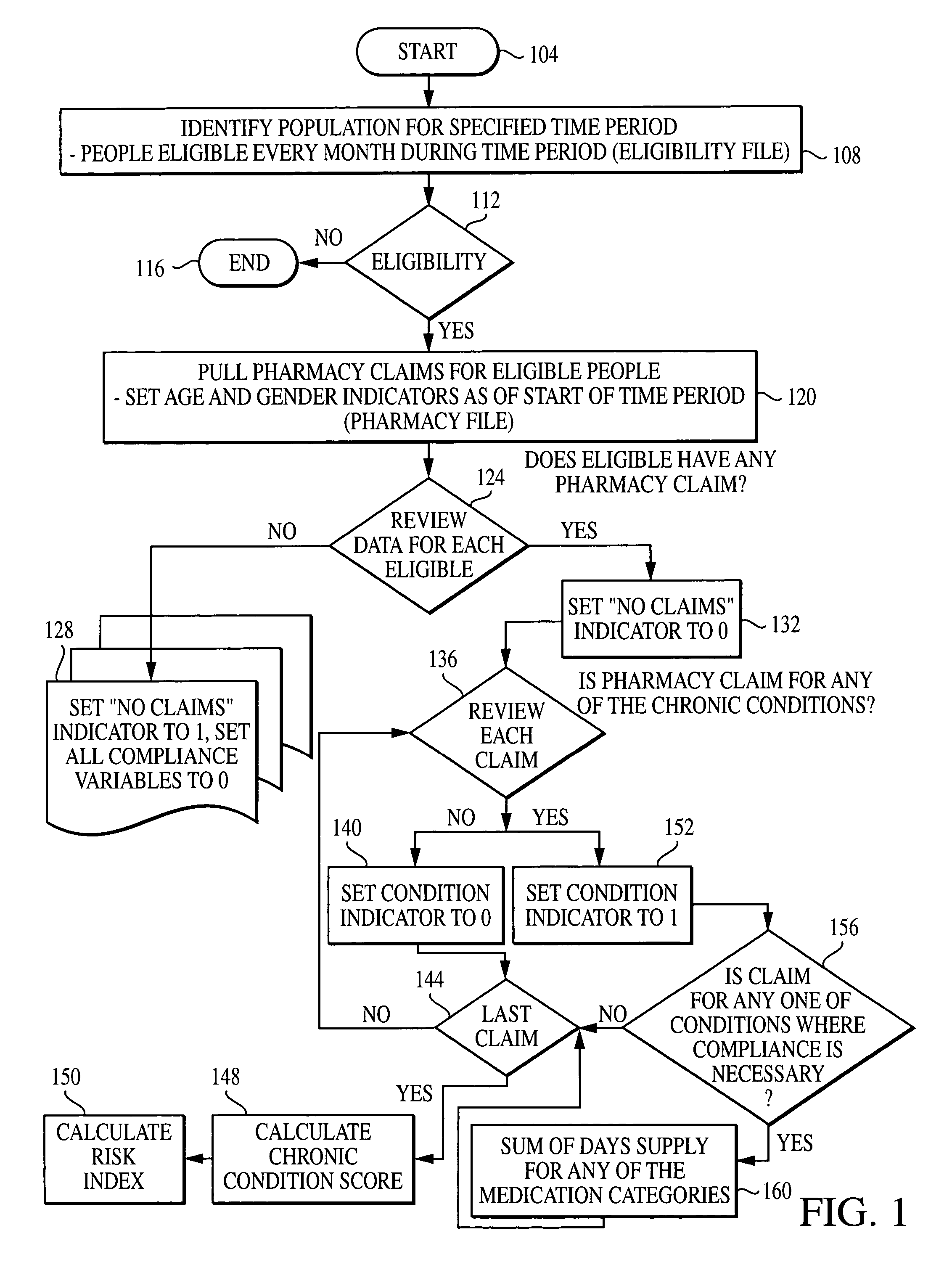
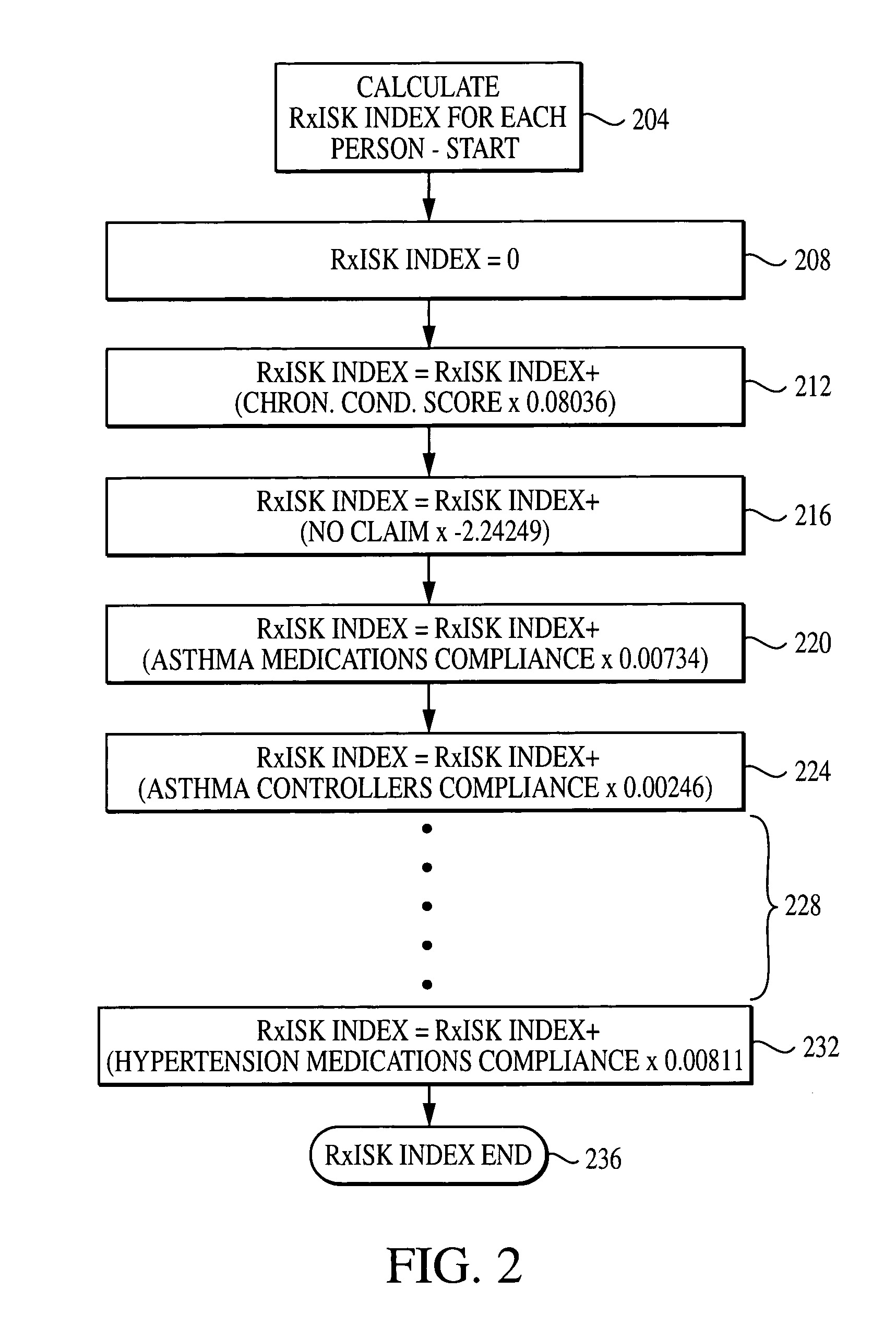
Computer system and method for generating healthcare risk indices using medication compliance information
A healthcare risk index is generated using a patient or individual's pharmacy claims. The index may be used to explain and predict variation in pharmacy-related costs and variation in total healthcare costs or utilization. In particular, the index is generated by first examining the individual's pharmacy claims to identify any chronic conditions possessed by the individual. Similarly, the individual's pharmacy claims are examined to identify any compliance medications prescribed to the individual. The chronic condition information is used to generate a chronic condition score by summing regression coefficients for each chronic condition possessed by the individual. Likewise, the compliance medication information is used to generate a compliance medication score by summing products of regression coefficients for each compliance medication prescribed to the individual with associated medication supply weights. From there, a modified chronic condition score is generated by multiplying the chronic condition score by an overall chronic condition regression coefficient. The modified chronic condition score may then be further modified by subtracting a no-claims weight from the chronic condition score in cases where the individual has no pharmacy claims. Finally, the risk index may be determined by summing the modified chronic condition score and the compliance medication score.
Owner:EXPRESS SCRIPTS STRATEGIC DEV INC
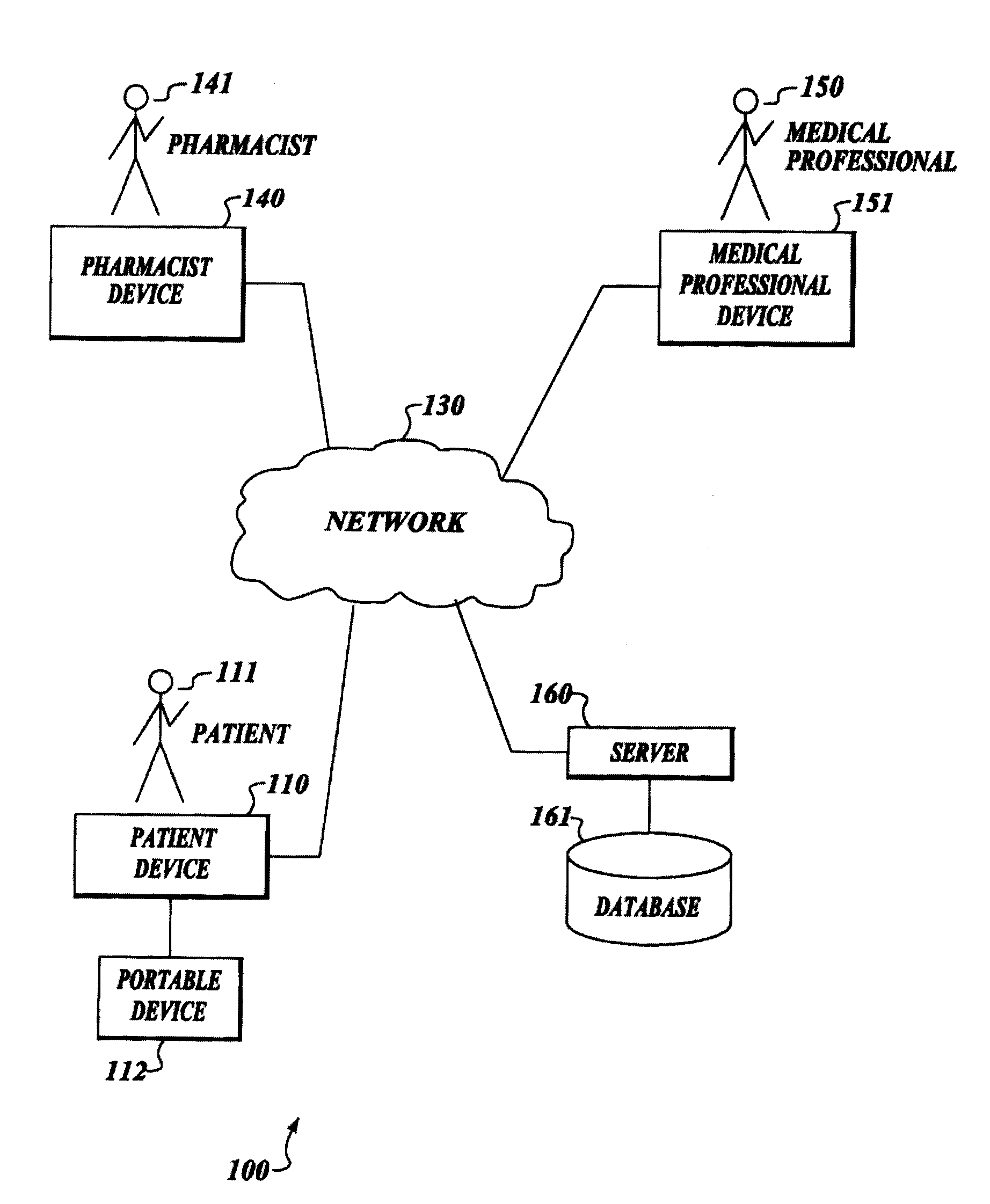
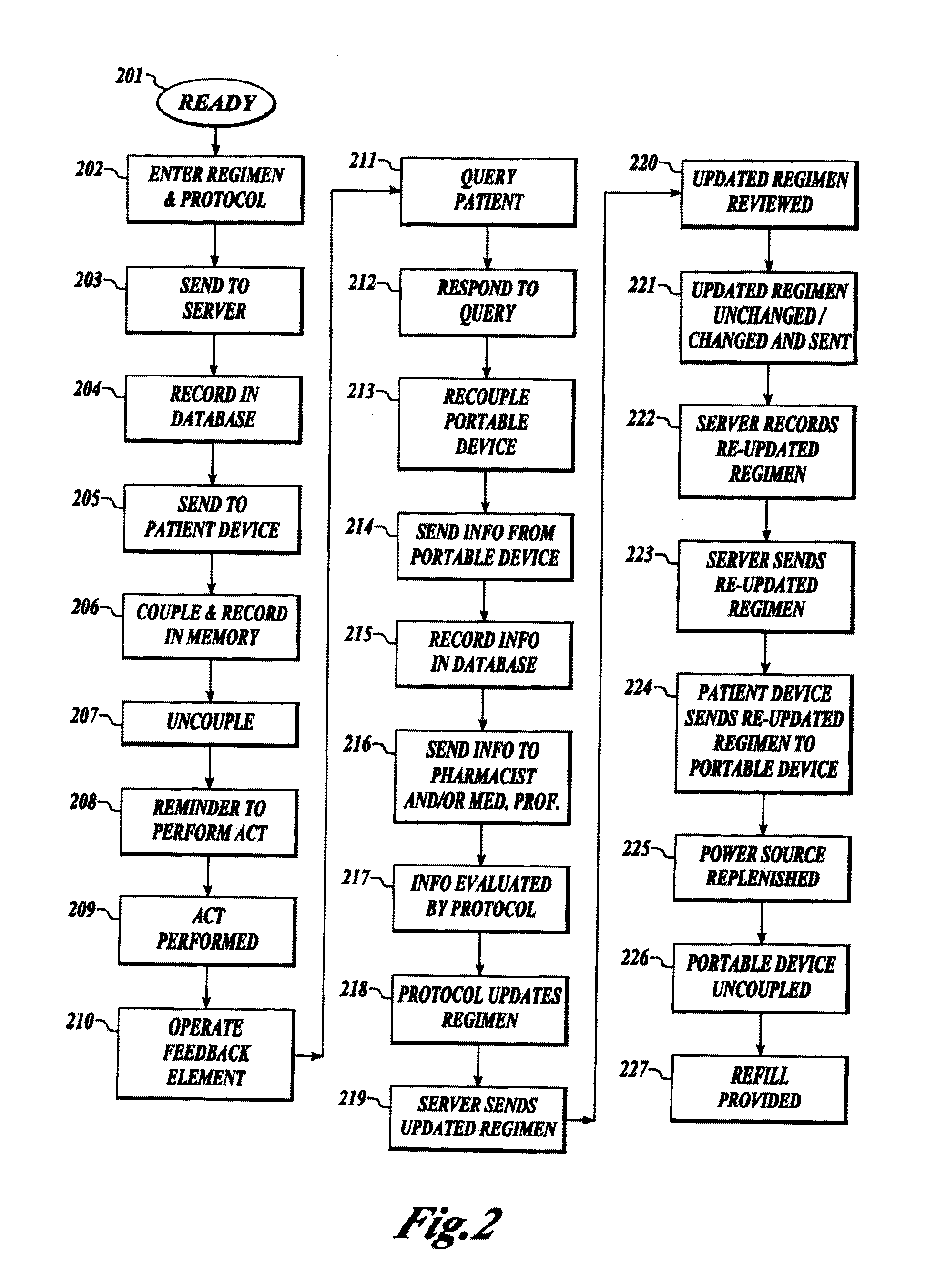
Treatment regimen compliance and efficacy with feedback
InactiveUS20080200771A1Maximize effectivenessData processing applicationsLocal control/monitoringMedicineCurative effect
A method and system for interaction with a community of individuals, relating to compliance with and effectiveness of treatment regimens, including supply and use of pharmaceuticals, using a protocol or other intelligent message which acts in place of a service provider and which is capable of collecting or imparting information to patients in place thereof. Individuals interact with the protocol or intelligent message to provide assistance in all aspects of treatment regimen compliance, data collection, supply or delivery, review and modification.
Owner:HEALTH HERO NETWORK
Compositions for weight management
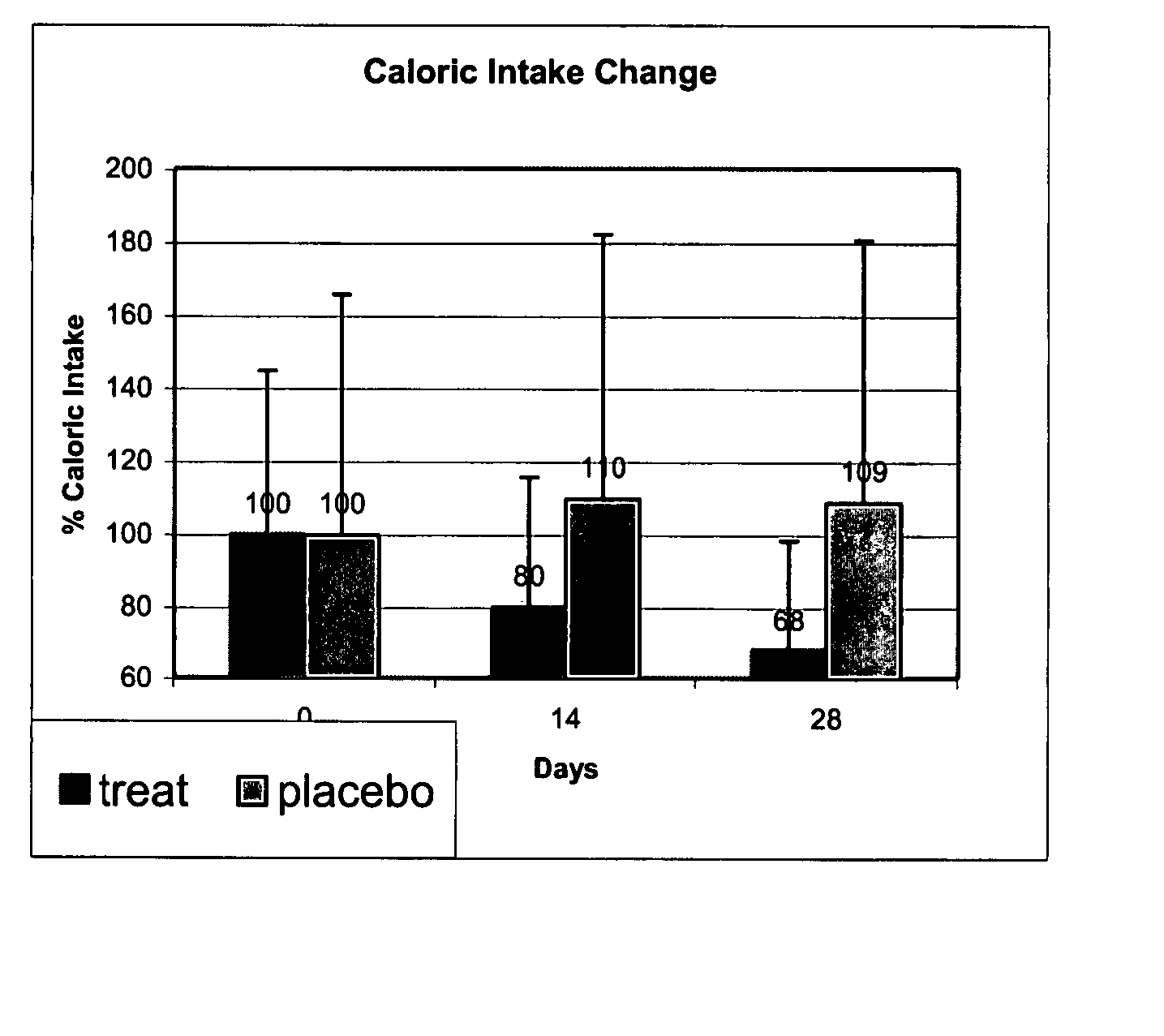
InactiveUS20080051440A1Prevent and reduce weight gainImprove efficiencyBiocideMetabolism disorderDyslipidemiaCompulsive eating
Methods and compositions for regulating food intake in a human subject; for improving a compliance of a human subject to caloric restriction; and for reducing a desire of a human subject to consume fats, utilizing H1-receptor agonists that have a pharmacological half-life that allows am efficient treatment regime thereof are disclosed. The methods and compositions can be efficiently used for treating conditions such as overeating, overweight, obesity, binge eating disorder, night eating syndrome, obsessive eating, compulsive eating and bulimia, as well as conditions associated with metabolic derangement such as dyslipidemia and for preventing or reducing weight gain due to factors such as drug use, cessation of smoking, and the like in a human subject.
Owner:MOR RES APPL LTD
Points-Based Reward Program For Improving Medication Adherence and Outcomes
Owner:PHARMMD SOLUTIONS
Anastrozole dispersed tablet formulation
InactiveCN1634042APromote absorptionImprove Medication AdherenceOrganic active ingredientsPill deliveryClinical efficacyClinical trial
The invention belongs to medical technology, in particular to a dispersible tablet form of anastrozole. Through a large number of clinical investigations, we have overcome the technical prejudice that anastrozole does not need to be developed into dispersible tablets in the prior art, and obtained the present invention. Clinical experiments have confirmed that the adverse reactions of the present invention are significantly reduced, and the compliance of medication is significantly improved. Obtained unexpected clinical efficacy. It has the characteristics of low production cost, stable preparation, fast absorption and high bioavailability. The invention is suitable for treating advanced breast cancer of postmenopausal women, and provides a good therapeutic drug dosage form for breast cancer patients.
Owner:LUNAN PHARMA GROUP CORPORATION
Roflumilast inhalation aerosol compound and preparation method thereof
InactiveCN104800214AReduce mouth sprayLong durationAerosol deliveryRespiratory disorderSide effectPoor adherence
The invention discloses a compound aerosol for quick-acting treatment of COPD, and a preparation method thereof. The compound aerosol comprises roflumilast and salbutamol. The compound aerosol combines respective characteristics of the long-acting COPD medicine roflumilast and the quick-acting COPD medicine salbutamol, and realizes rapid effectiveness and long lasting time in order to reduce the oral spray frequency of patients and reduce toxic side effects. A propellent changes from CFCs to HFC in order to eliminate destroys to the earth's ozone layer. Lecithin selected in the invention is in favor of realizing immersion and penetration of medicines to lung cells in order to improve the bioavailability and realize quick acting. Mentholum is added into a dispersant, so the patients have refreshing and comfortable feeling after administration, thereby the patients' compliance is improved.
Owner:成都英诺新科技有限公司
Low-carbon cold-rolled steel plate for enamel and production method thereof
ActiveCN109943779AExcellent anti-scale explosion performanceExcellent elongationSheet steelChemical composition
The invention discloses a low-carbon cold-rolled steel plate for enamel and a production method thereof, and belongs to the technical field of metal materials. The low-carbon cold-rolled steel plate for enamel disclosed comprises the following chemical components in percentage, by mass, 0.010% to 0.030% of C, Si is less than or equal to 0.0030%, 0. 10% to 0.40% of Mn, P is less than or equal to 0.015%, 0.010% to 0.030% of S, 0.010% to 0.60% of Als, 0.010% to 0.040% of Ti, 0.0010% to 0.0070% of N, and the balance of Fe and inevitable impurities. The method aims to overcome the defects that in the prior art, the defects that the cold-rolled steel plate often generates scale explosion, poor adherence and the like in the enamel process are overcome, the low-carbon cold-rolled steel plate for enamel and a production method of the low-carbon cold-rolled steel plate for enamel has good anti-scale explosion performance, adherence property and pinhole resistance after enamel.
Owner:MAANSHAN IRON & STEEL CO LTD
Medicine for treating dumps emotional handicap type disease
InactiveCN101081272AEasy to takeImprove compliancePowder deliveryNervous disorderDiseaseTypes diseases
The medicine for treating depression and other mental disturb diseases is prepared with chlorimipramine, sulpiride, sodium valproate, borneol and Chinese herbal medicine extractum prepared with nutagrass flatsedge rhizome, arisaema with bile, wild jujube seed and other Chinese medicinal materials. The medicine consists of chemically synthetic medicine and Chinese herbal medicine component, and has synergistic treating effect, less toxic side effect, and good patient's compliance.
Owner:张宝山
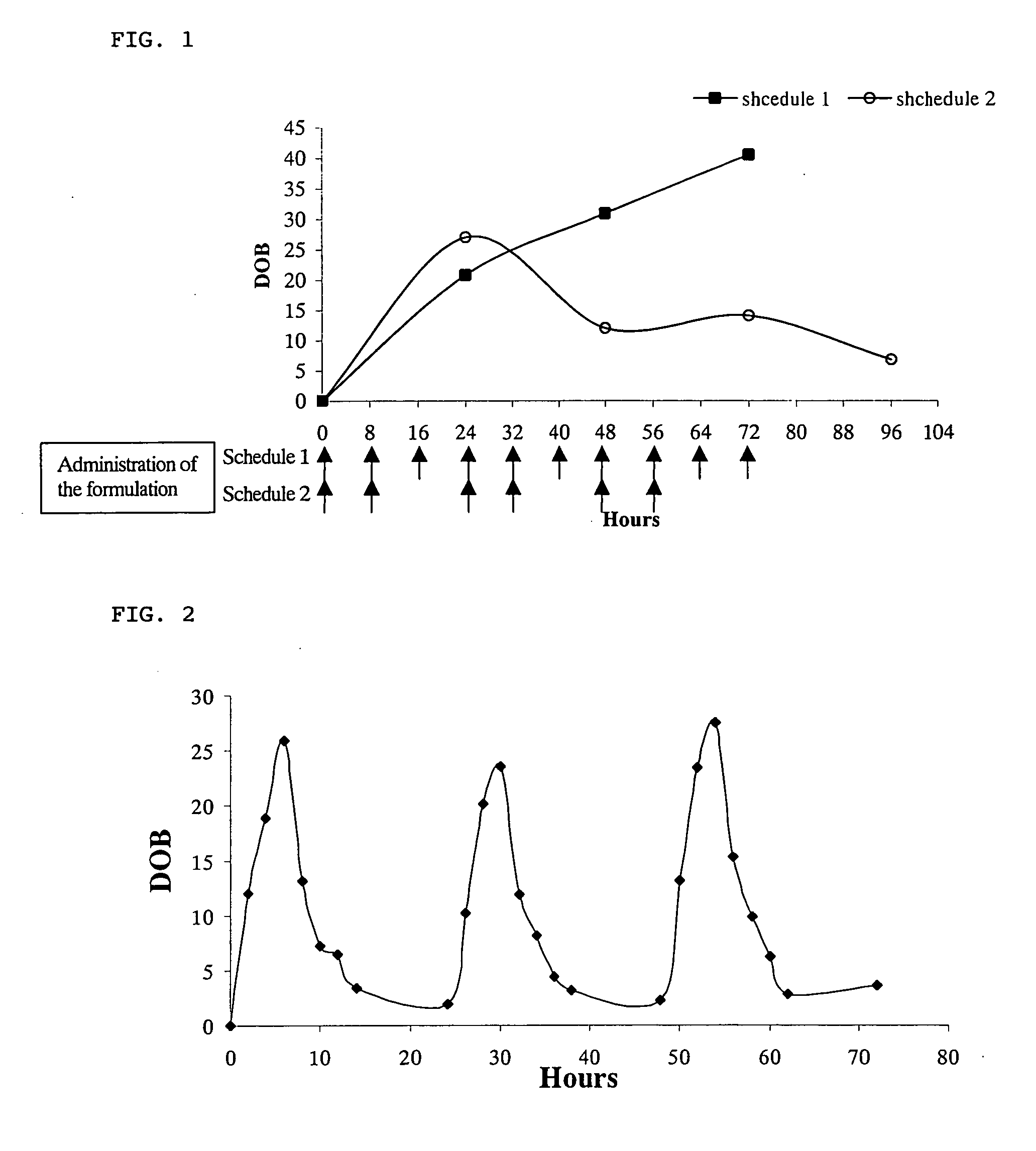
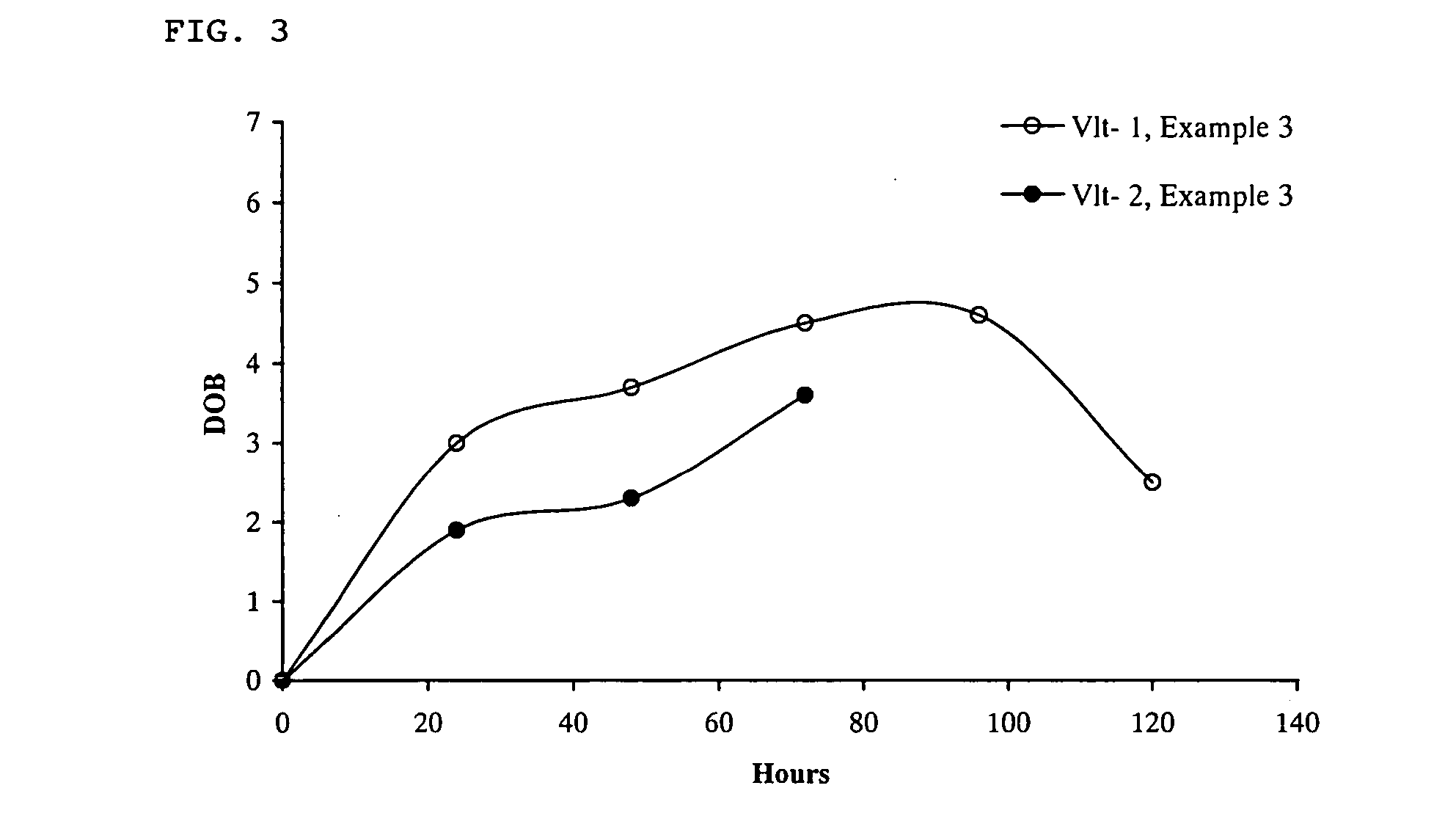
Method for monitoring patient or subject compliance with medical prescriptions, and formulation for use in the method
InactiveUS20060188444A1Easily accurately monitoringBig burden to solveVaccination/ovulation diagnosticsDiagnostic recording/measuringLipid formationMedicine
The present invention provides a highly accurate method for monitoring patient or subject compliance with a medication prescription by collecting a sample of the exhaled air. The method of the present invention is easy and little burdens the patient or subject. The method comprises the steps of: (i) prescribing a patient or subject ingestion of at least one biologically active agent and at least one carbon isotope-labeled lipid, (ii) obtaining a sample of the patient's or subject's exhaled air, (iii) measuring the ratio of the carbon isotope-labeled CO2 relative to 12CO2 in the sample; and (iv) confirming the ingestion of the prescribed biologically active agent based on the ratio of the carbon isotope-labeled CO2 relative to 12CO2.
Owner:OTSUKA PHARM CO LTD
Methods and apparatus for dispensing medicines
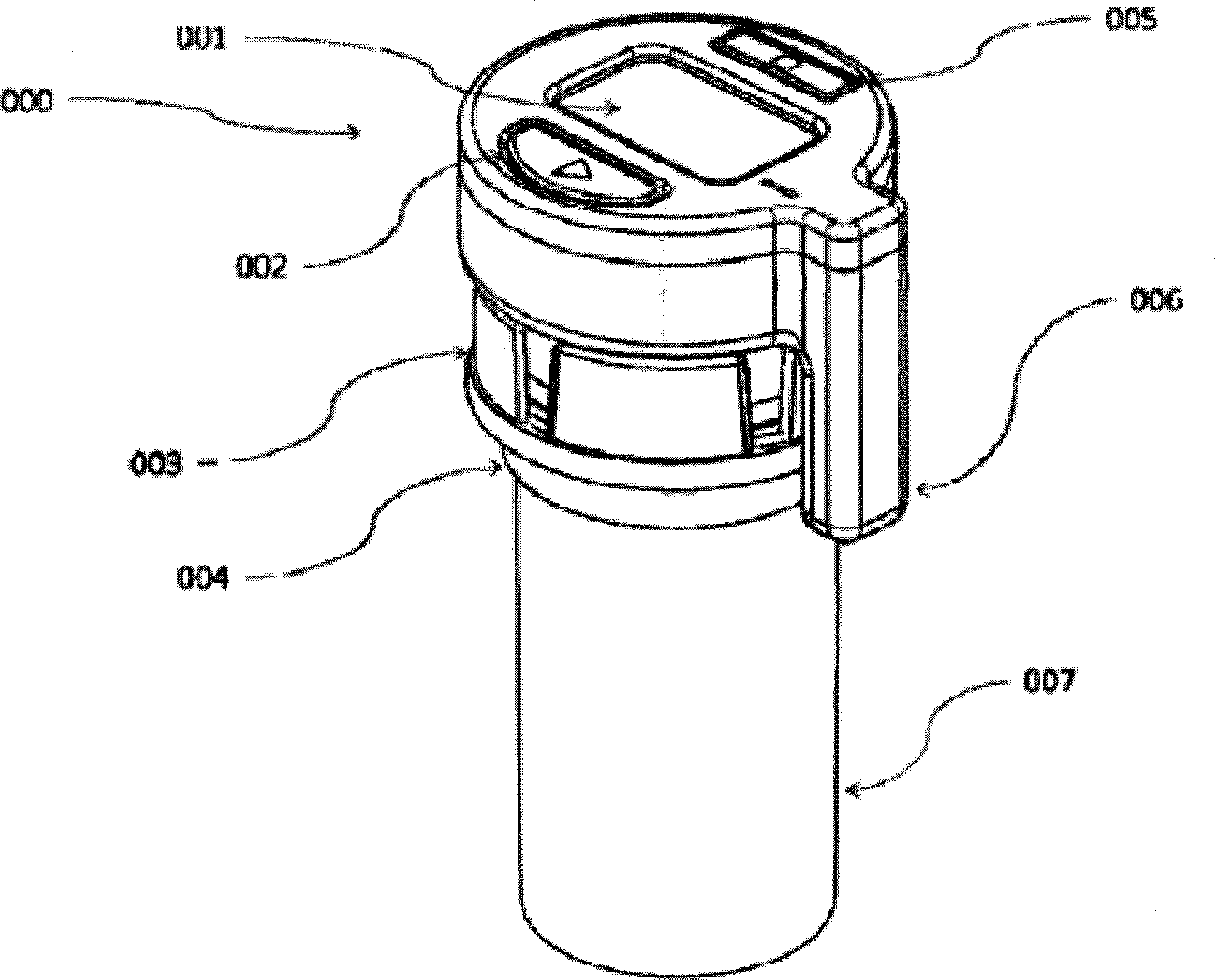
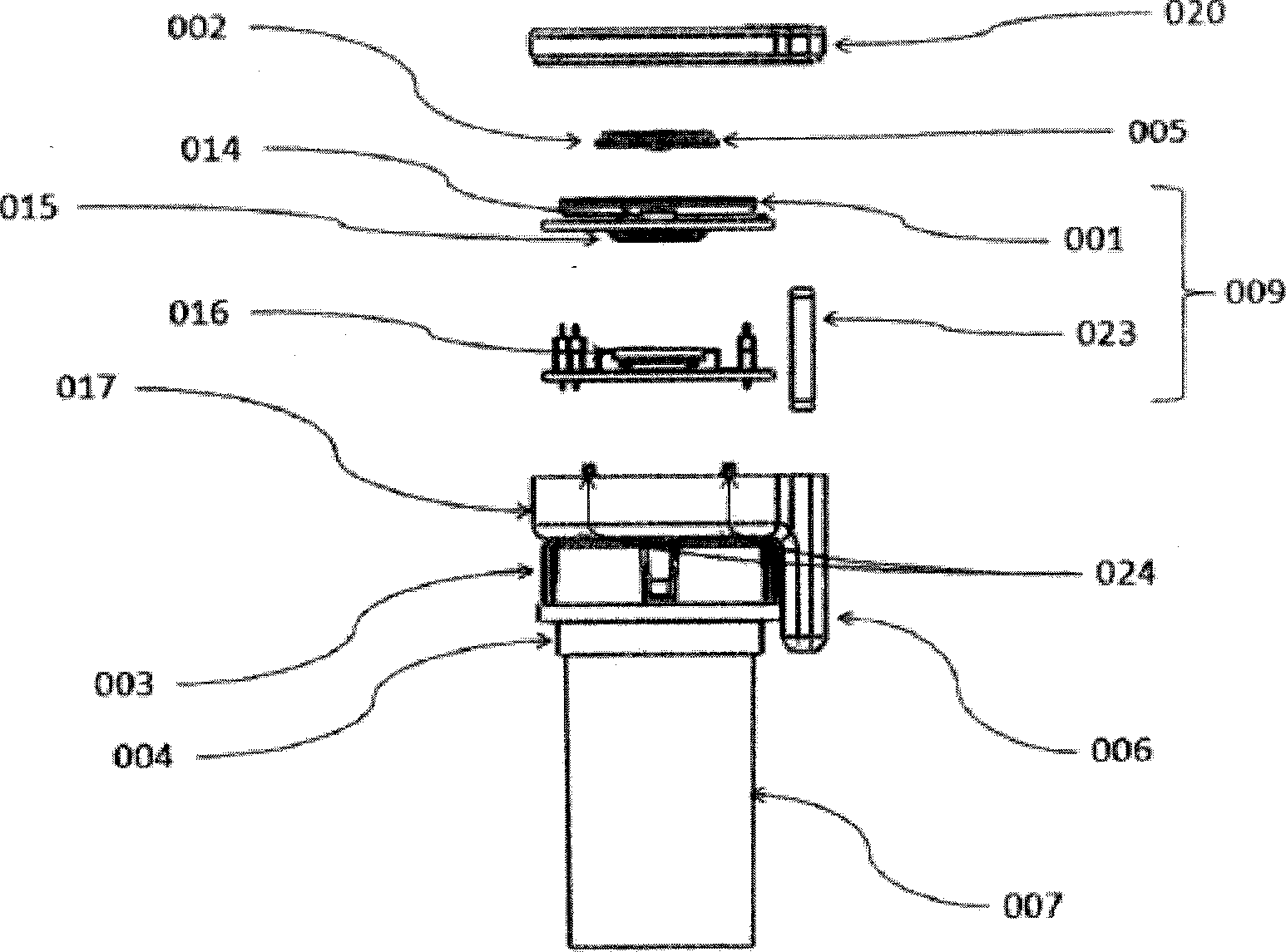
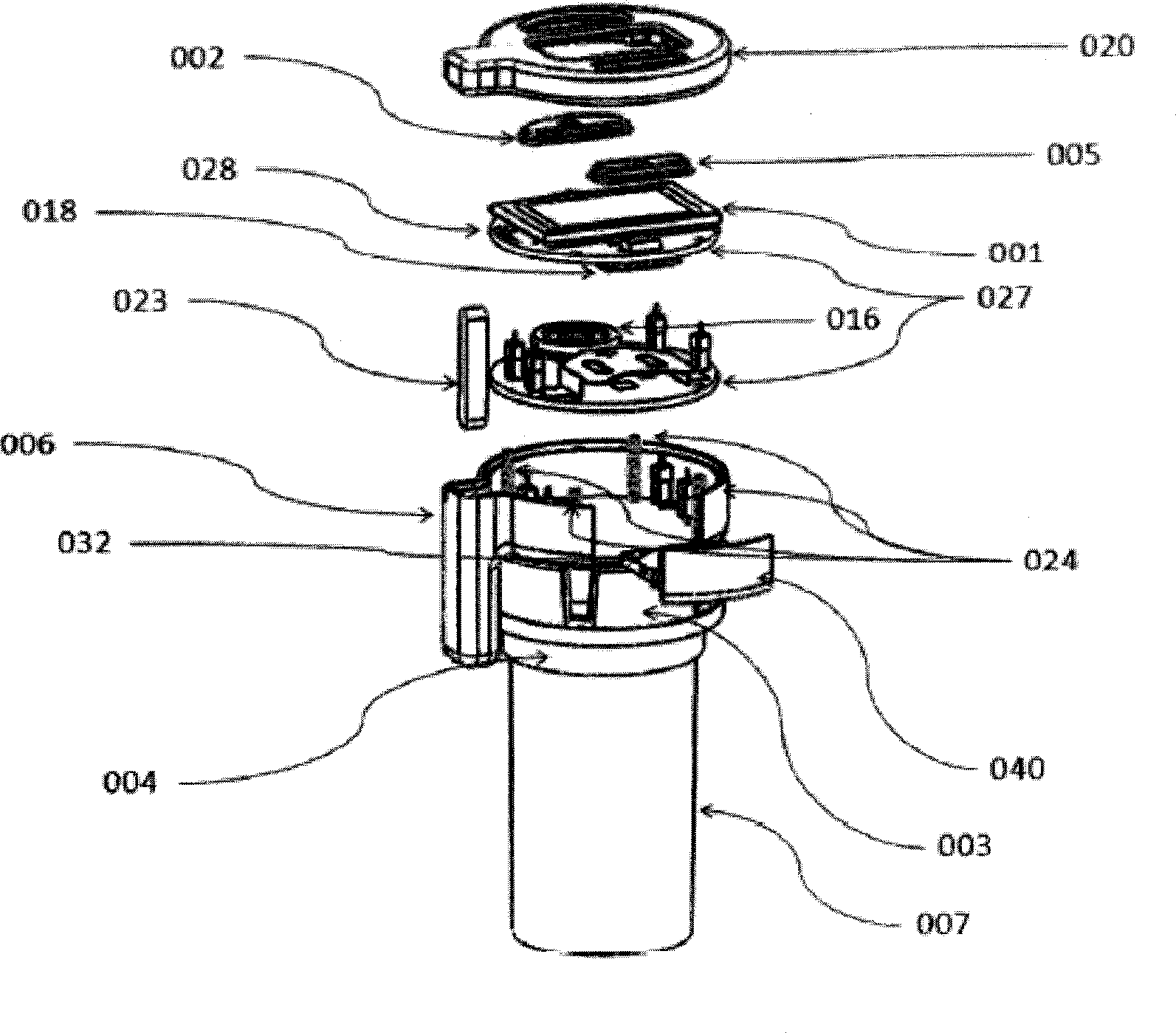
The present is directed to an integrated system and method for improving patient medication adherence. The device of the present invention provides reminders, instructions and tracking of medication ingestion, and is easily adaptable to fit on any medication vial cap.
Owner:许宁
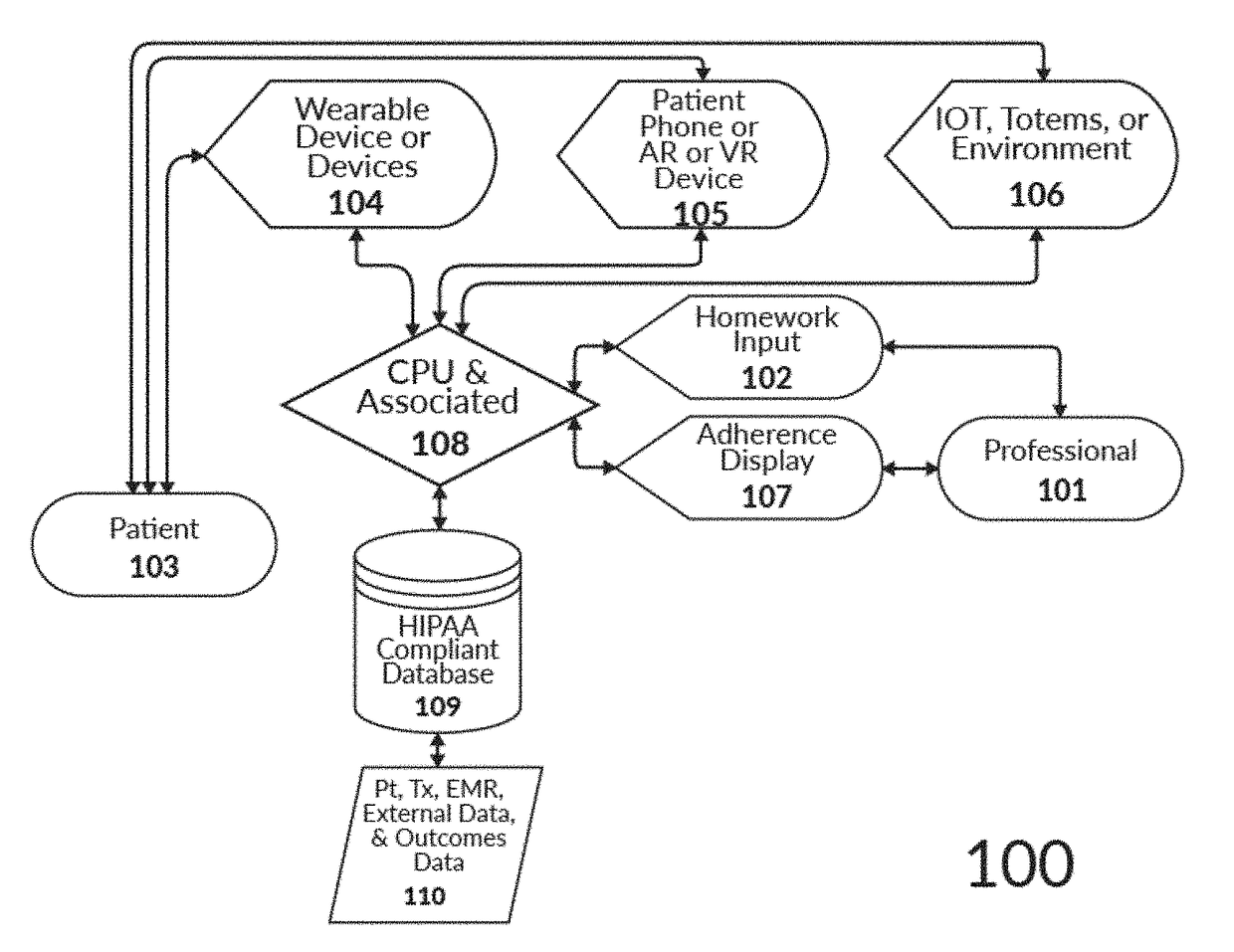
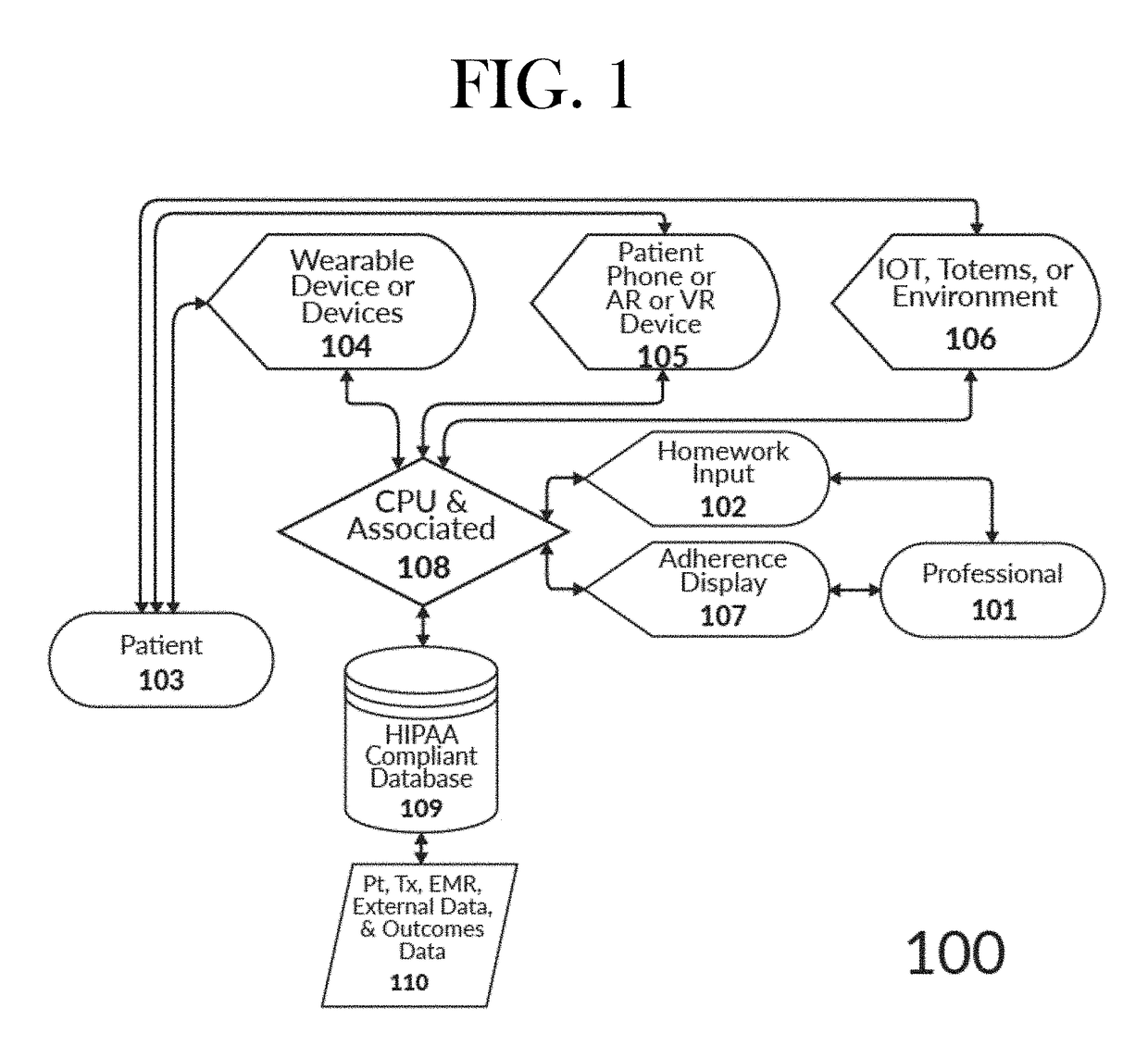
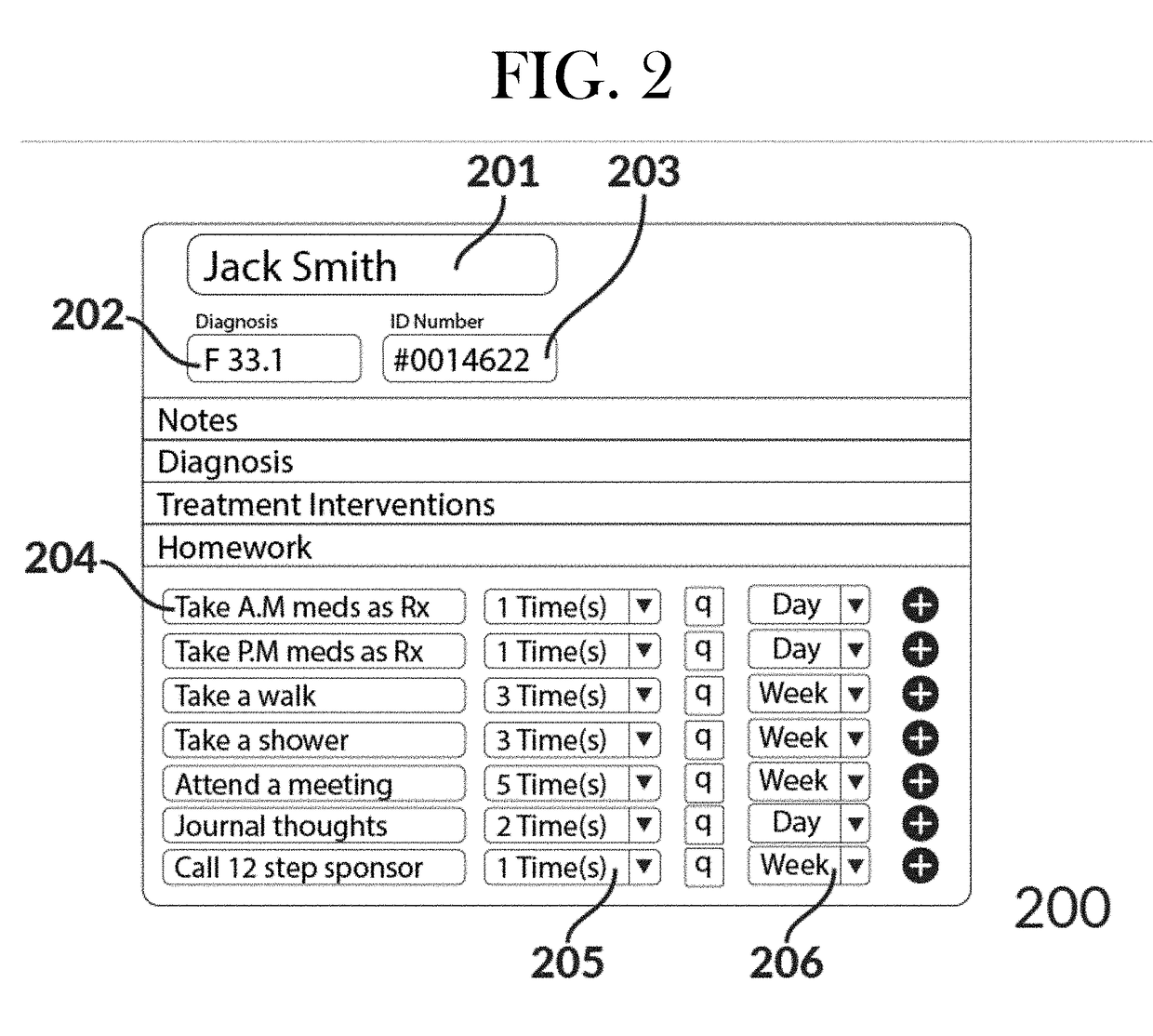
Intelligent mobile homework adherence and feedback application for telehealth
InactiveUS20170213006A1Improve recommendationsView accuratelyDrug and medicationsTelemedicineComputerized systemPoor adherence
The present invention relates to a computerized system for and methods of securely providing precision healthcare services related to the AI-optimized prescribing and monitoring of behavioral health homework at a distance. The system is continually learning in a recursive manner such that the output of one set of patient experiences are added to the AI system to further refine future patient recommendations with regard to prescribed homework assignments. The present invention is unlimited with regard to the type of patient entity or healthcare professional entity.
Owner:THE LIVE NETWORK INC
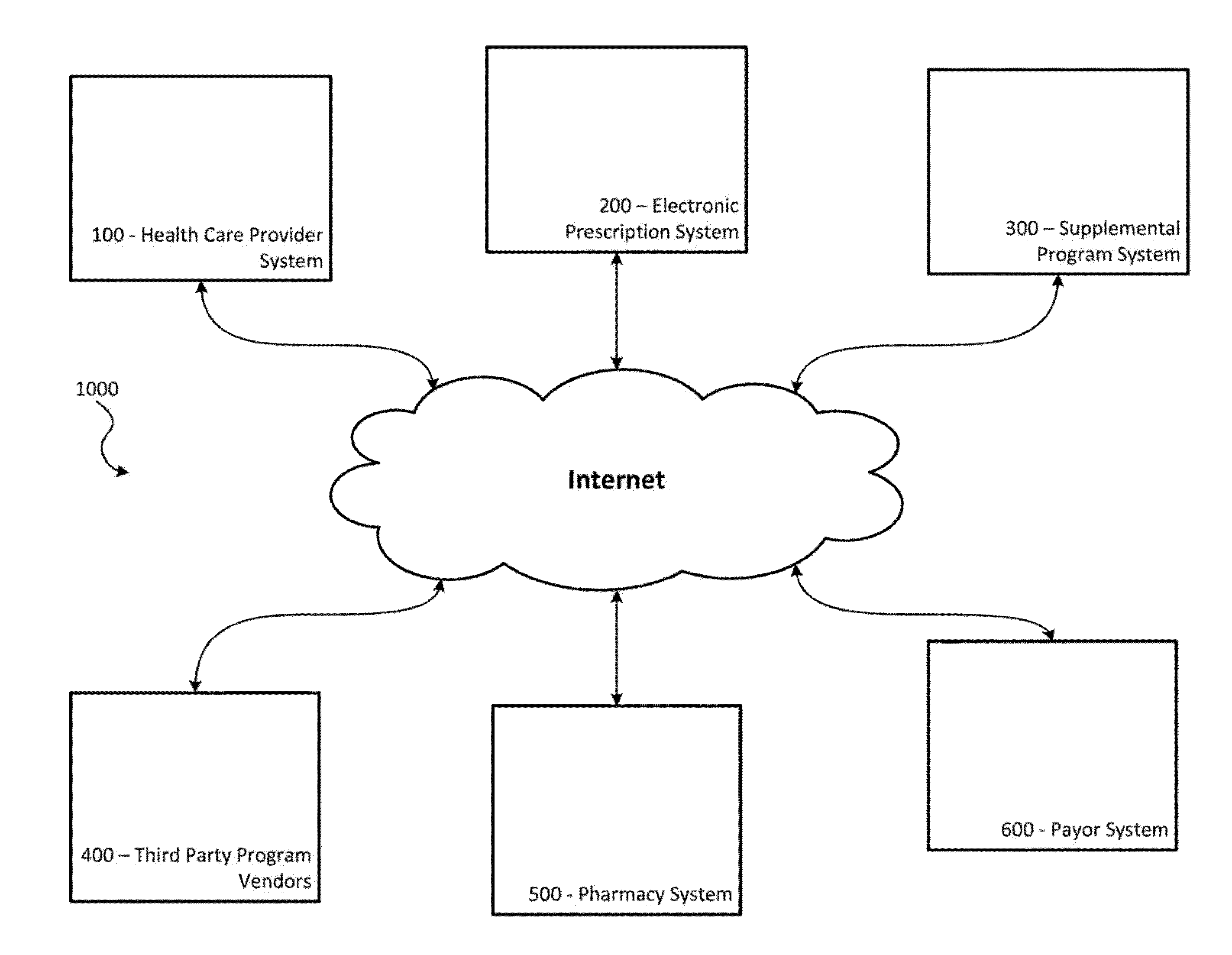
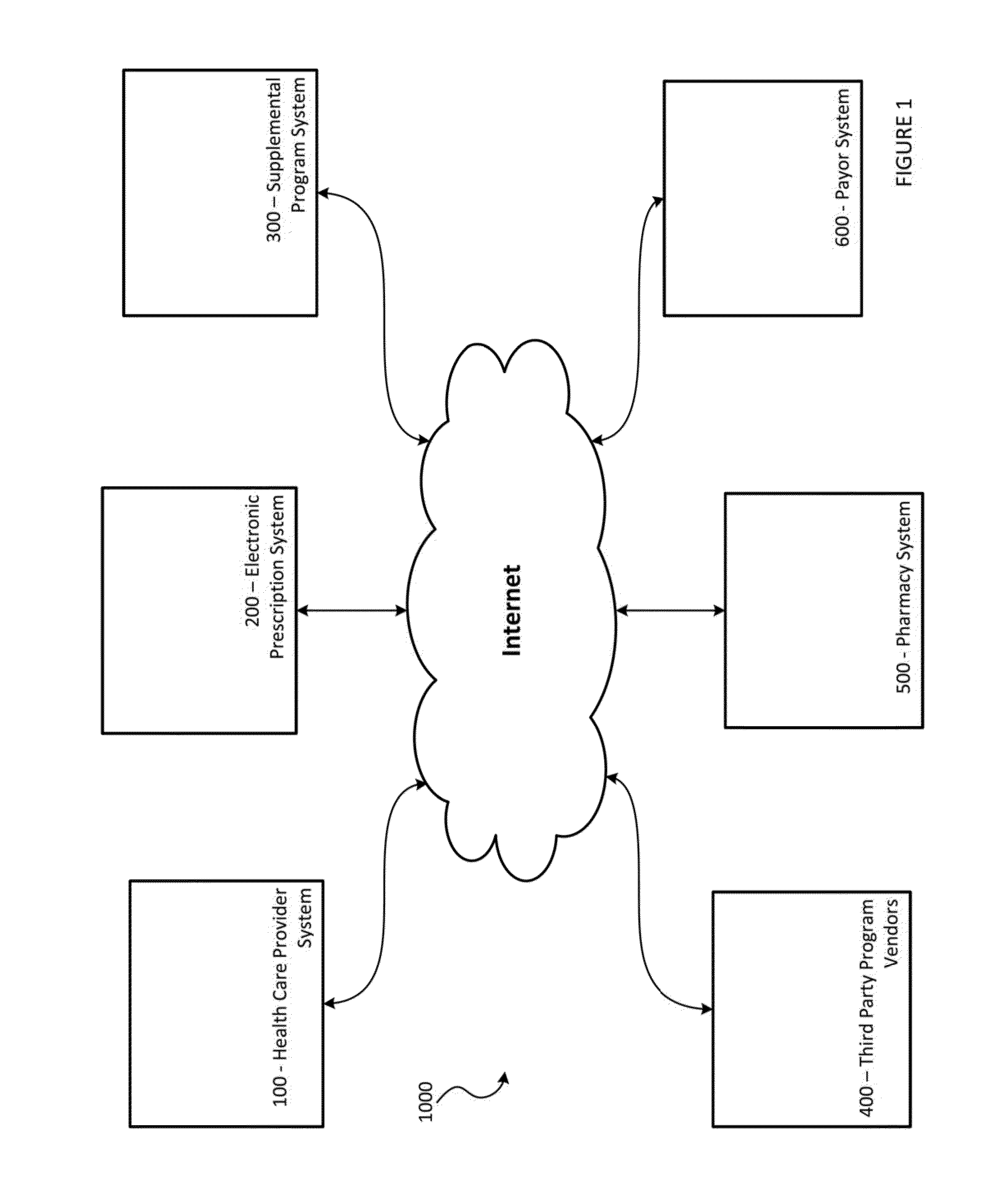
Systems and Methods for Supplementing Patient and Provider Interactions to Increase Patient Adherence
The present invention relates generally to a method of supplementing an electronic prescription issued by a health care provider, the method comprising: a) receiving, on a computer apparatus, electronic prescription data generated by a health care provider for a patient for a prescribed substance; b) the computer apparatus determining, from a plurality of available supplemental programs stored on one or more databases, supplemental programs for which the patient is eligible based on the electronic prescription data; c) presenting to the health care provider, in a display device, a list of the eligible supplemental programs, each of the eligible supplemental programs being selectable and de-selectable by the health care provider in the display device; and d) the computer apparatus activating each supplemental program from the plurality of available supplemental programs that have been selected and confirmed by the health care provider in the display device.
Owner:DRFIRST COM INC
Analytics regarding patient care
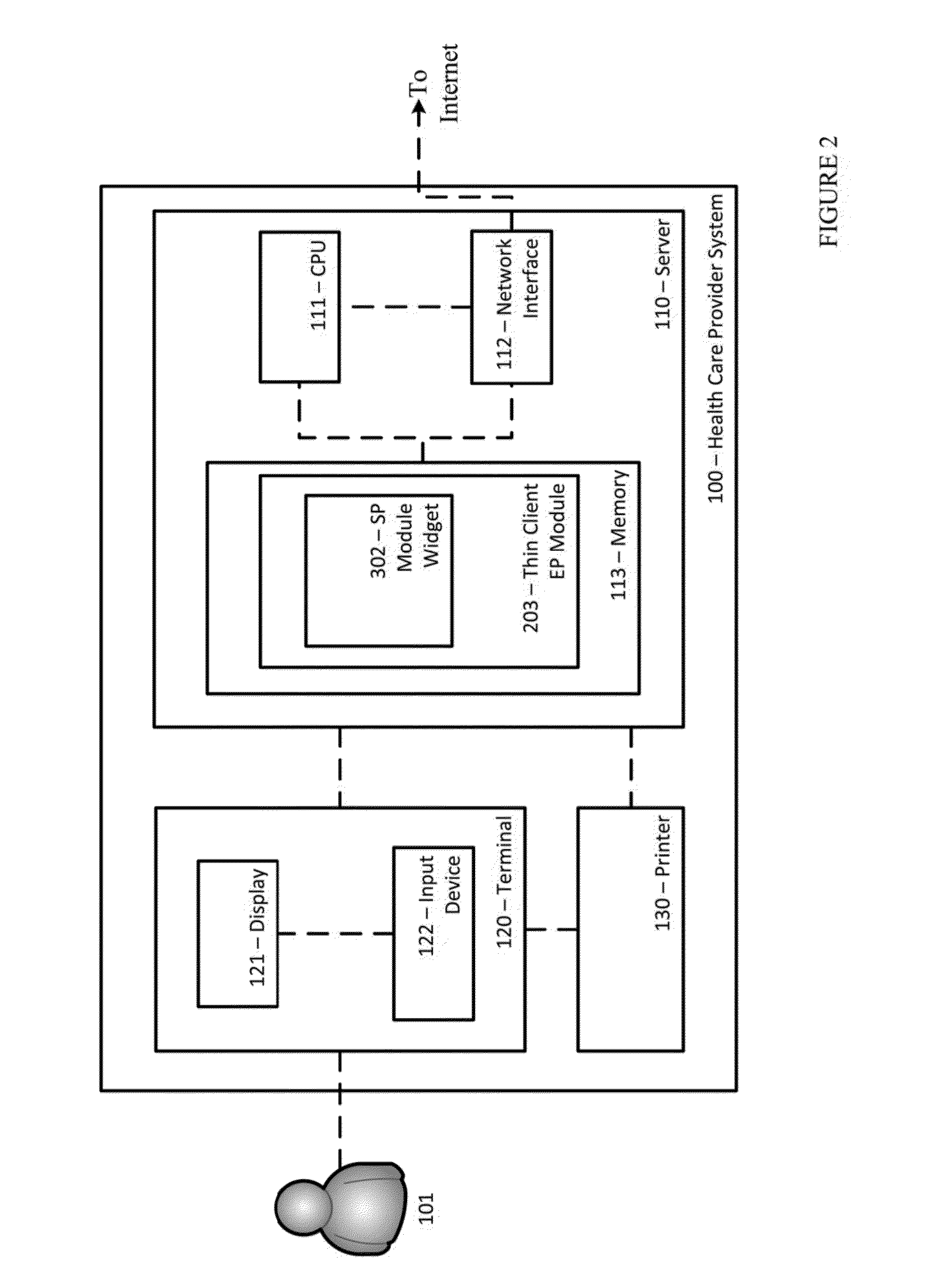
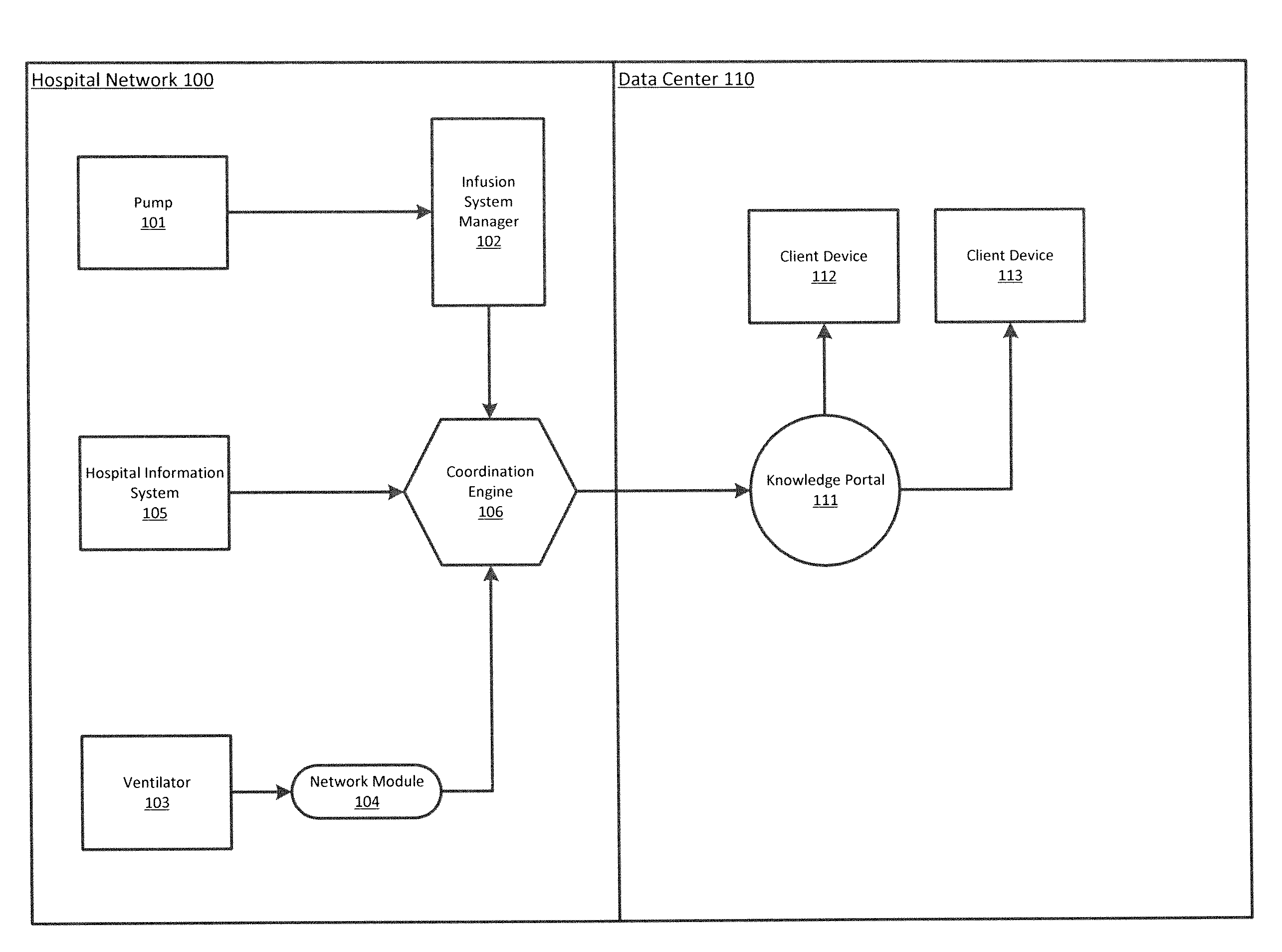
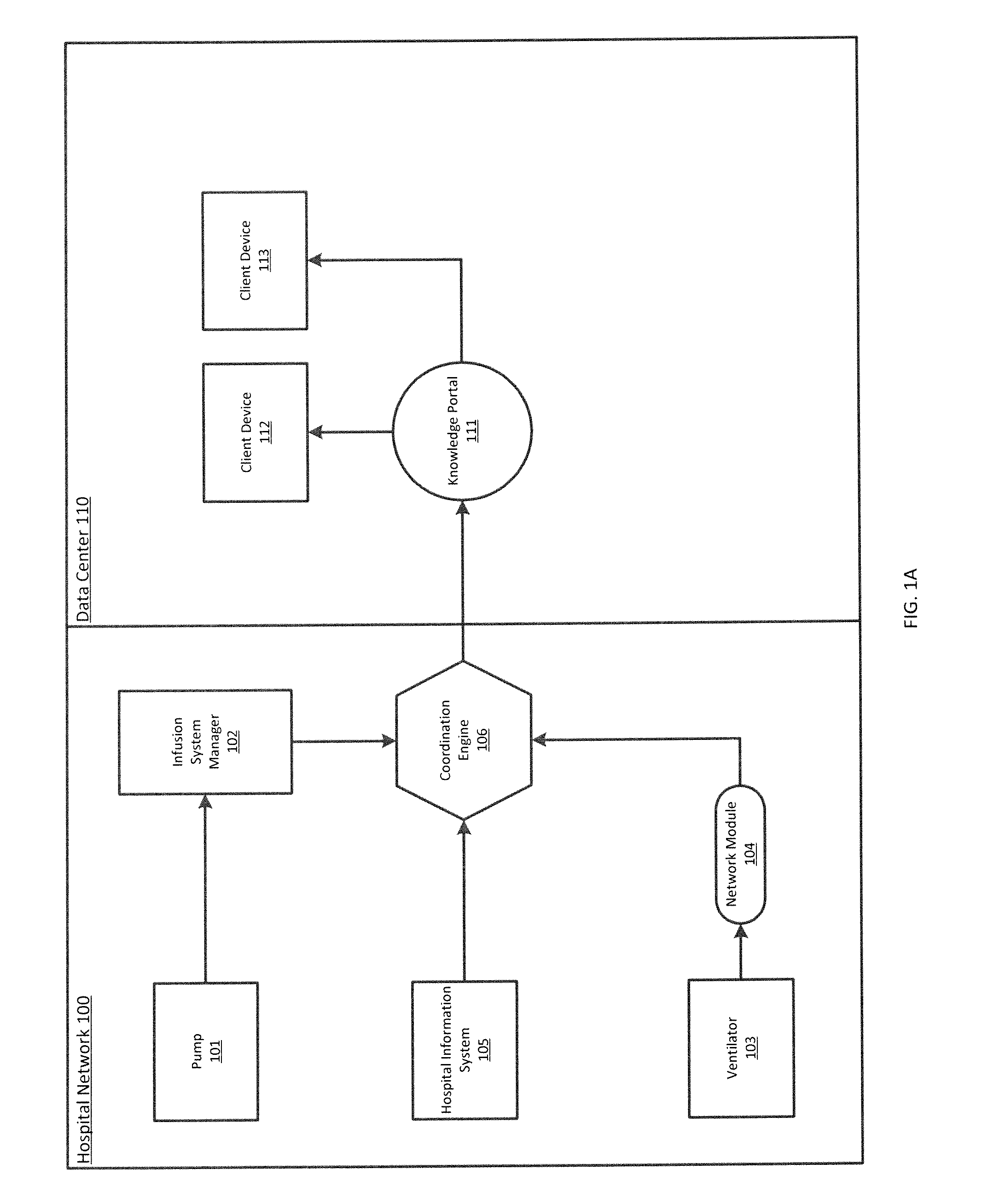
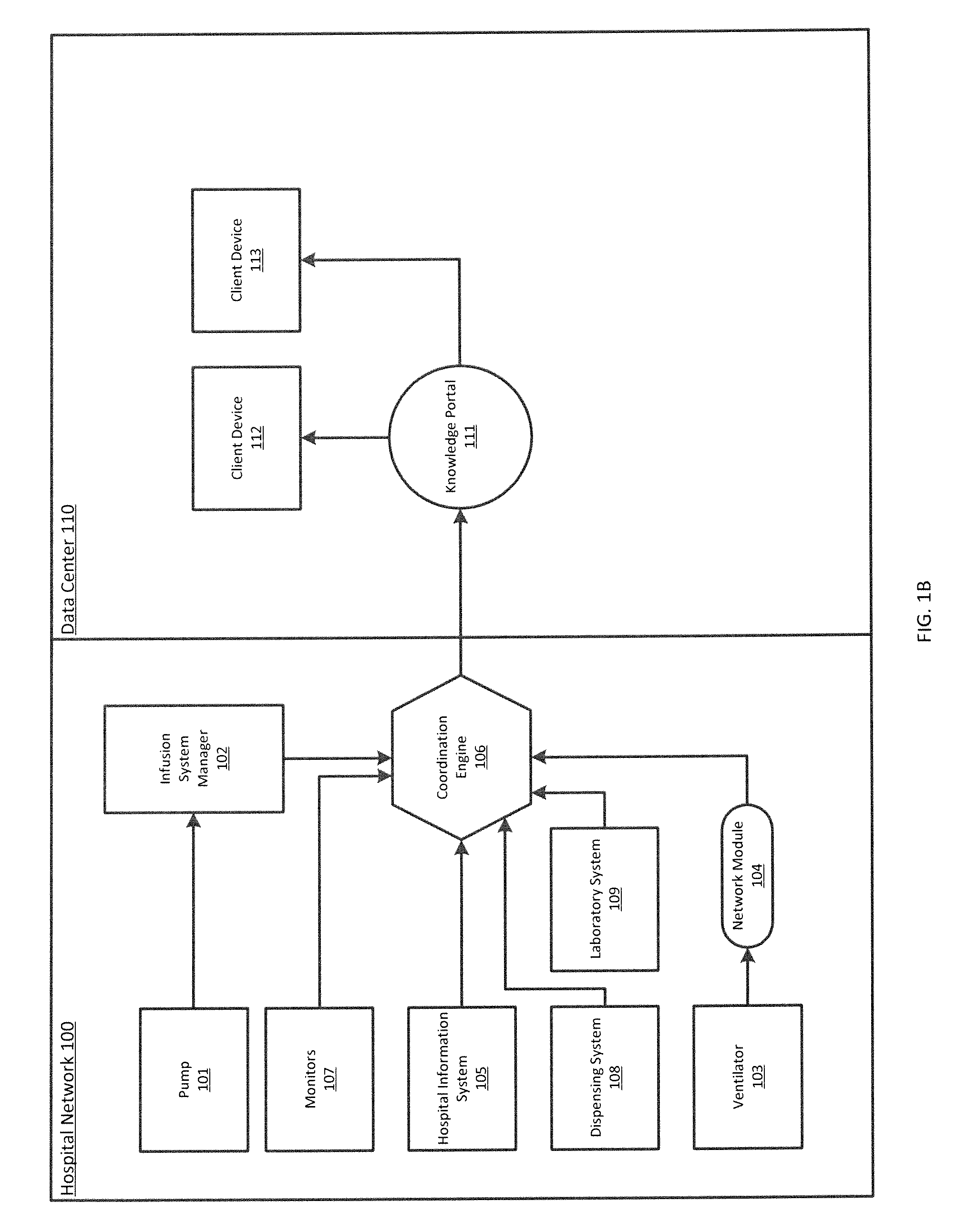
ActiveUS20140371543A1Facilitate managing rule and protocolRespiratorsDrug and medicationsPoor adherenceInfusion pump
A method, system and computer-readable medium are provided for determining compliance with patient care protocols, comprising an infusion pump providing infusion information pertaining to one or more drugs administered to a patient, the one or more drugs including sedatives and analgesics, and a processor communicably coupled to the infusion pump and configured to determine information regarding the one or more drugs being administered to the patient based on the infusion information by determining a baseline threshold for the one or more drugs being administered to the patient according to the actual dosage of the one or more drugs administered to the patient prior to a current time, determining a dosage amount of the one or more drugs currently being administered to the patient and comparing the dosage amount of the one or more drugs currently being administered to the baseline threshold to determine a deviation from the baseline threshold.
Owner:CAREFUSION 303 INC
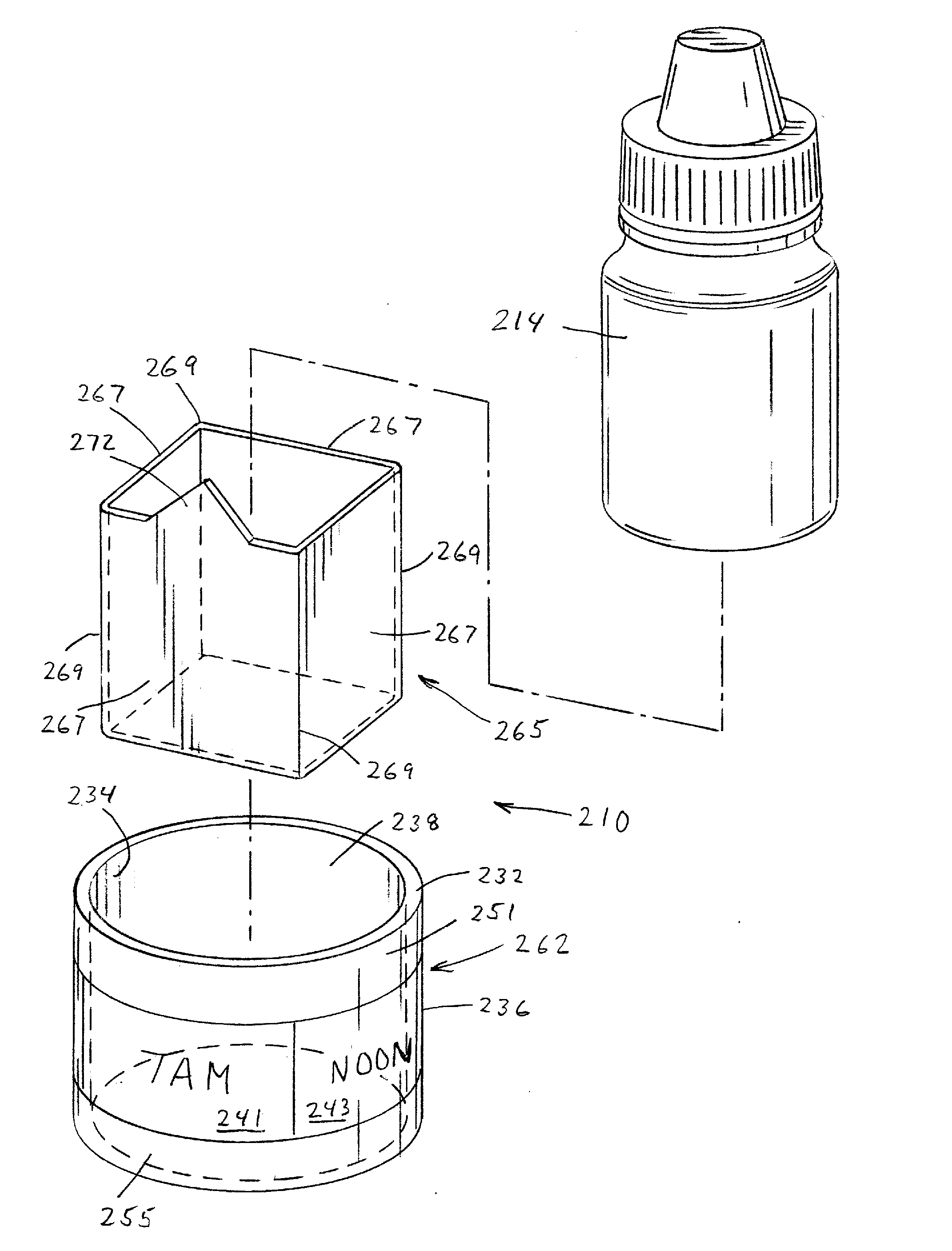
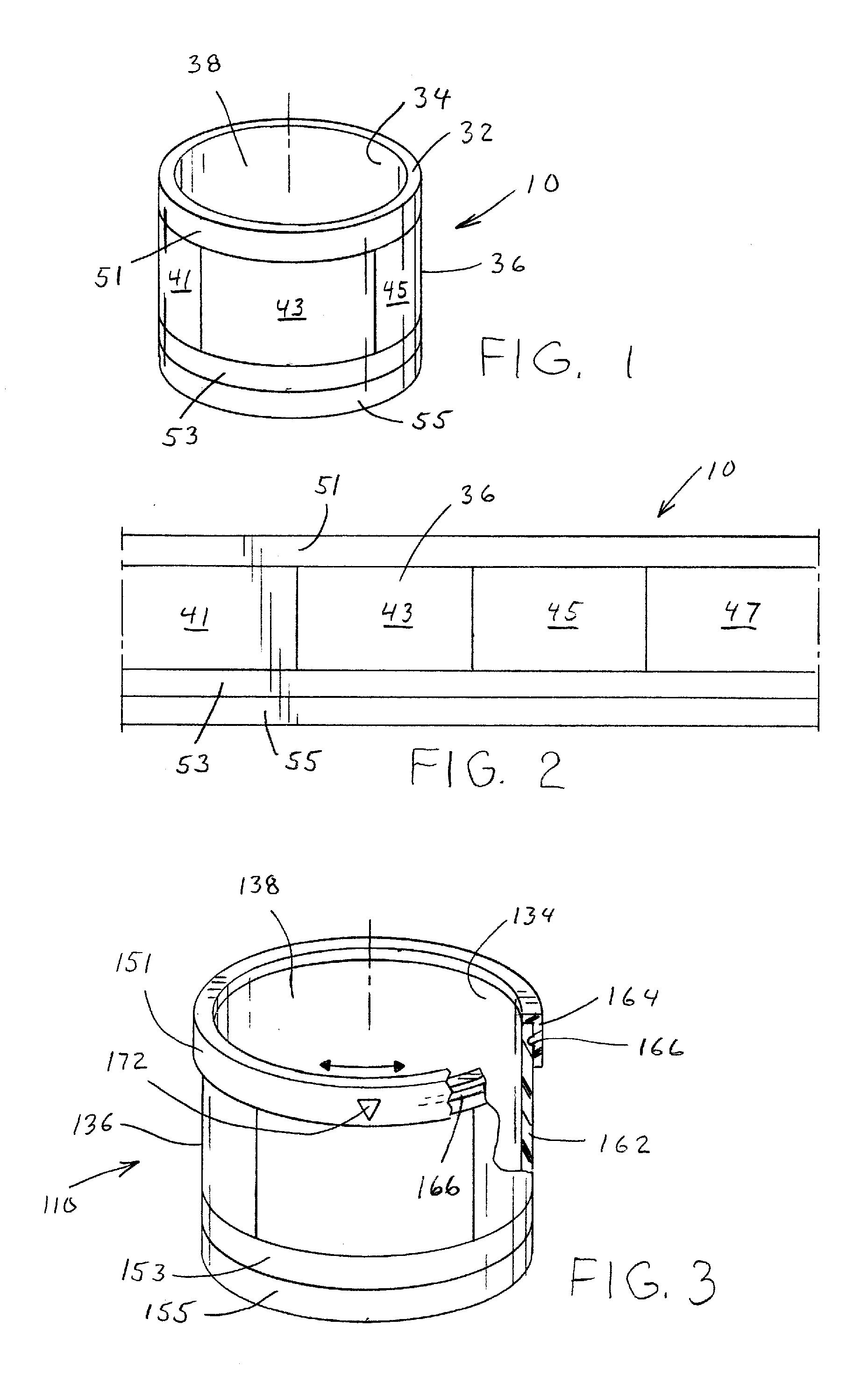
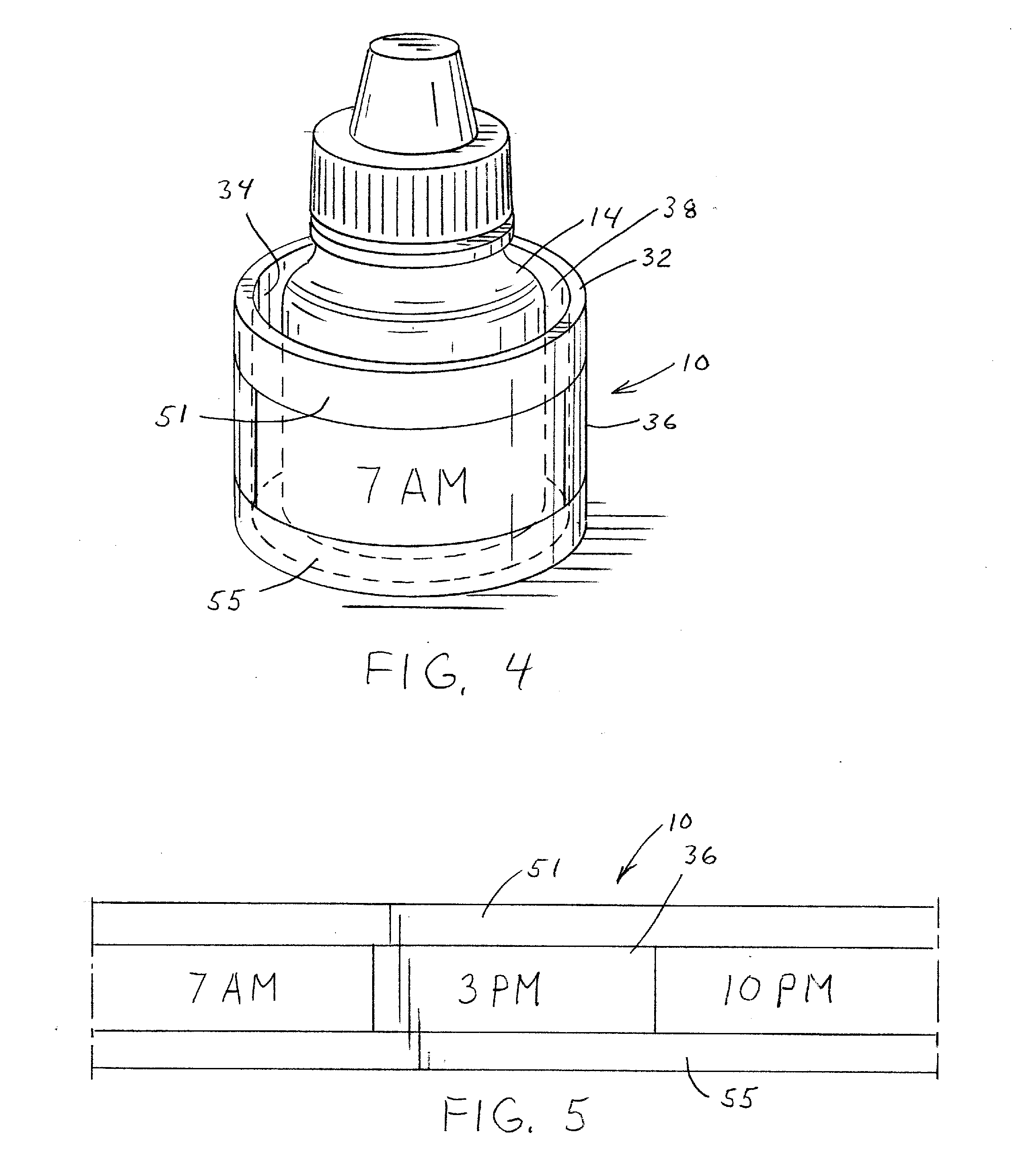
Medication adherence reminder device
InactiveUS20140155841A1Improve prescription medication complianceImprove persistencyBottlesPharmaceutical containersMedication adherenceMedicine
The present invention provides a device for tracking medical dosage schedules. The device includes a generally tubular device that has an inner surface and an outer surface, wherein the outer surface includes visual indicia related to dosing of medication wherein the device is configured to be positioned around a container of the medication. By positioning the device relative to the container, the user can determine which dose has been administered and which dose is next to be administered.
Owner:DOSSIN CHRISTOPHER J
Internet-based patients' medicine taking intervention method
InactiveCN107978375ARealize daily medication reminderCheck in timeDrug referencesDiseaseDrug withdrawal
Owner:HARBIN GUANGKAI TECH DEV
Polyethylene composition for large-scale container lining
The invention relates a polyethylene composition for a large-scale container lining. The polyethylene composition for a large-scale container lining comprises polyethylene, modifiers and an anti-oxidant. Compared with the prior art, the polyethylene composition for a large-scale container lining has the advantages that through the synergism of the modifiers and the anti-oxidant which is a traditional anti-oxidant, polyethylene performances are improved; and through the improvement of polyethylene resin polarity and adherence between a metal framework and a polyethylene lining, a product service life is improved and thus the problem of poor adherence between the existing plastic lining and the metal framework is solved thoroughly.
Owner:CHINA PETROLEUM & CHEM CORP +1
New purpose of antithrombin III and kit
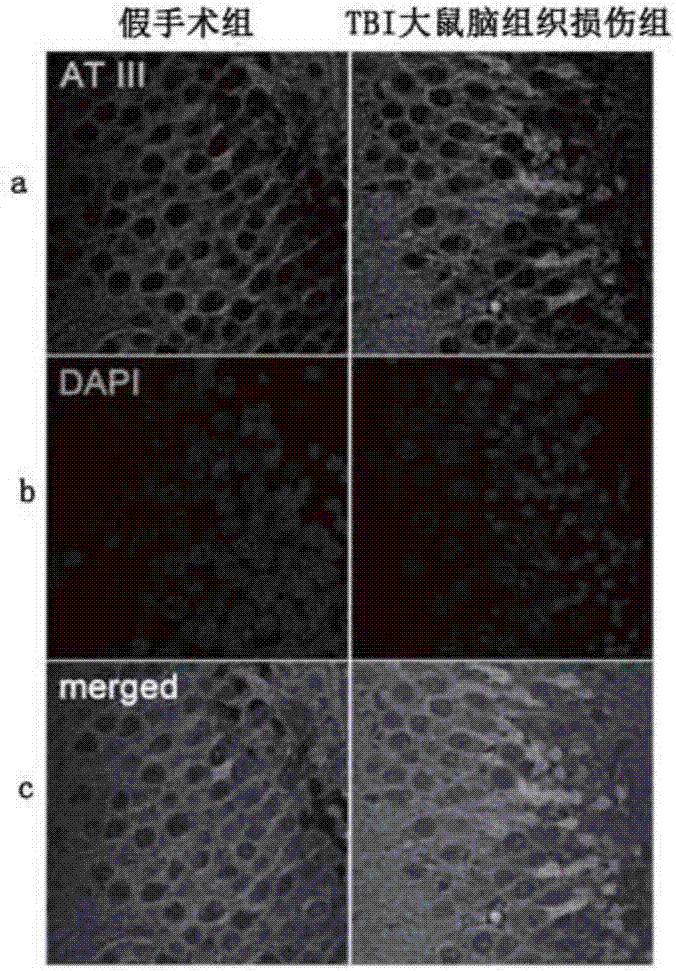
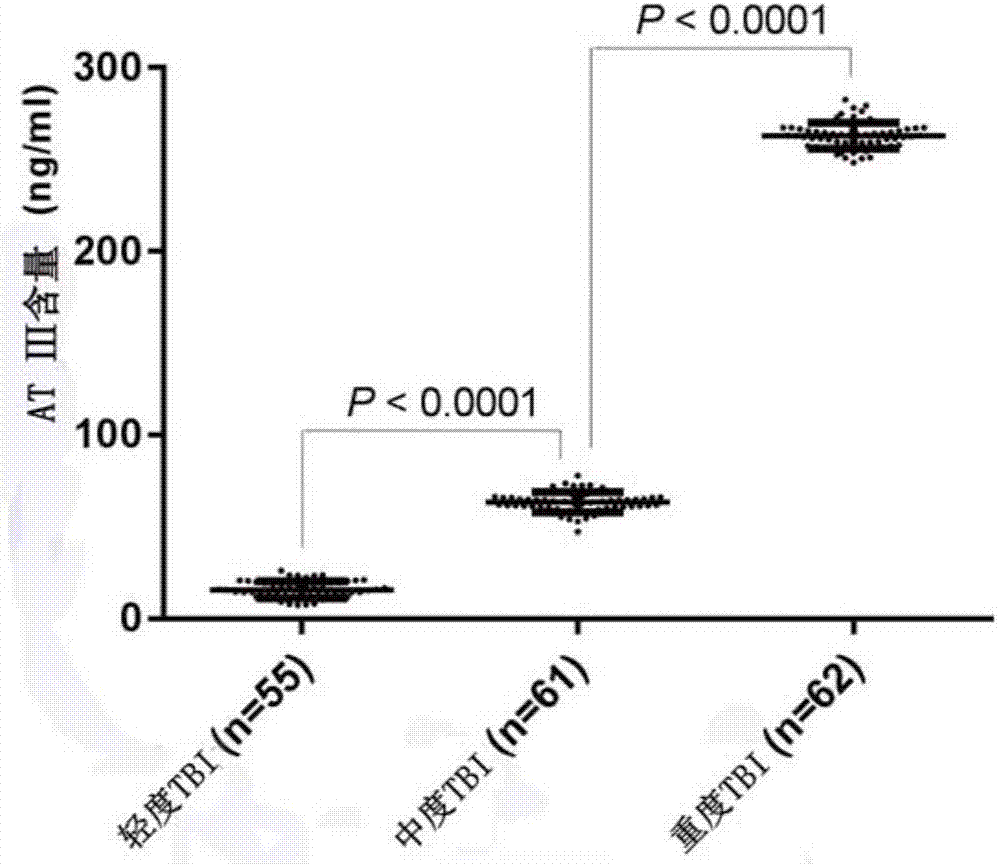
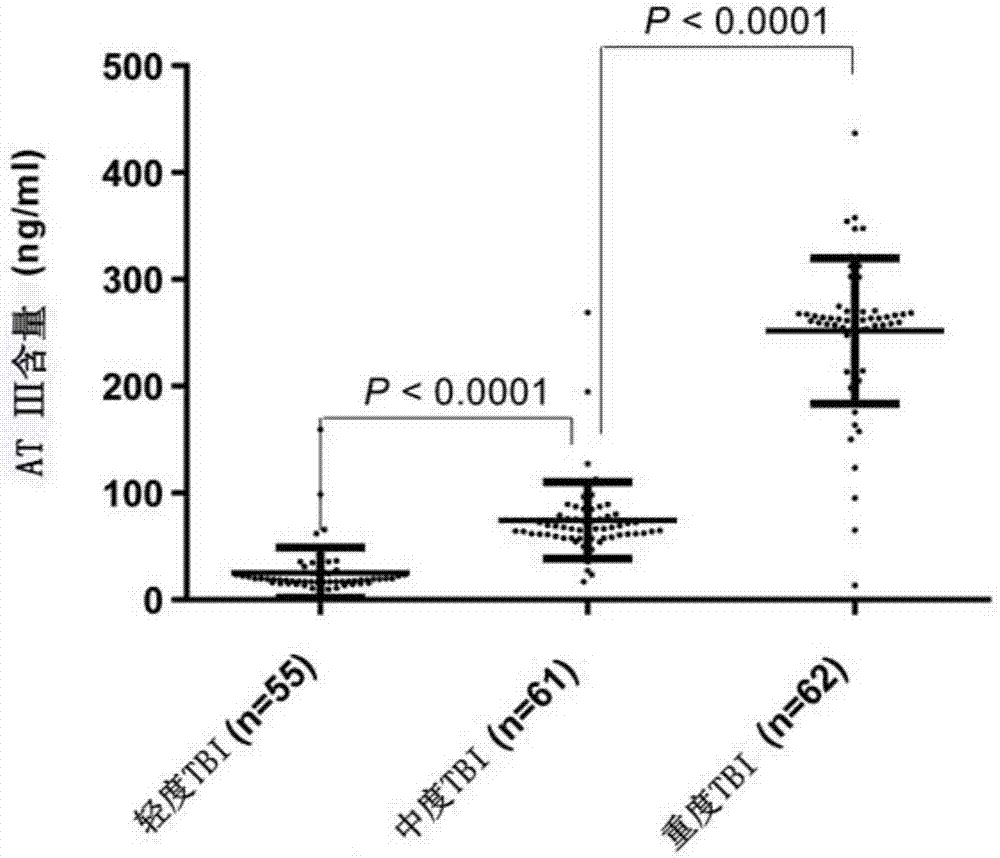
ActiveCN107576804AImprove complianceStrong specificityBiological testingSubstance usePatient compliance
The invention provides new purpose of antithrombin III and a kit, which have the benefits that a substance used for detecting the antithrombin III can be used for evaluating the injury state of a TBIpatient or used for detection and auxiliary detection on the severity of the injury state of the to-be-evaluated TBI patient; when being used for evaluating or detecting the severity of the injury state of the TBI patient, the method has the advantages of being good in specificity, high in sensitivity, good in reproducibility, good in patient compliance and the like. A test proves that the antithrombin III can be taken as a marker for evaluating the severity of the injury state of the TBI patient and can also be used for evaluating the severity of the injury state of the TBI patient; results are accurate, the operation is simple and convenient, and the application value is large.
Owner:唐颐生物医学(天津)有限公司 +1
Anastrozole dispersed tablet formulation
InactiveCN100337625CPromote absorptionImprove Medication AdherenceOrganic active ingredientsPill deliveryClinical efficacyAnastrozole
The present invention belongs to the medicine technology, especially an anastrozole dispersed tablet dosage form. Through plentiful clinical investigations, we conquer the technology prejudice in prior art that it is not necessary to exploit anastrozole into dispersed tablets and obtains the present invention. The clinical experiments approve that the adverse reaction in accordance with the present invention is remarkably reduced,the compliableness of said drug is remarkably improved, and unexpected clinical curative effects are acquired. Said medicine is characterized by its low fabricating cost, formulation stabilization, fast absorption, high bioavailability and the like. The present invention is suitable for treating advanced breast carcinoma of females after menopause and provides favorable therapeutic agents dosage form to breast carcinoma patients.
Owner:LUNAN PHARMA GROUP CORPORATION
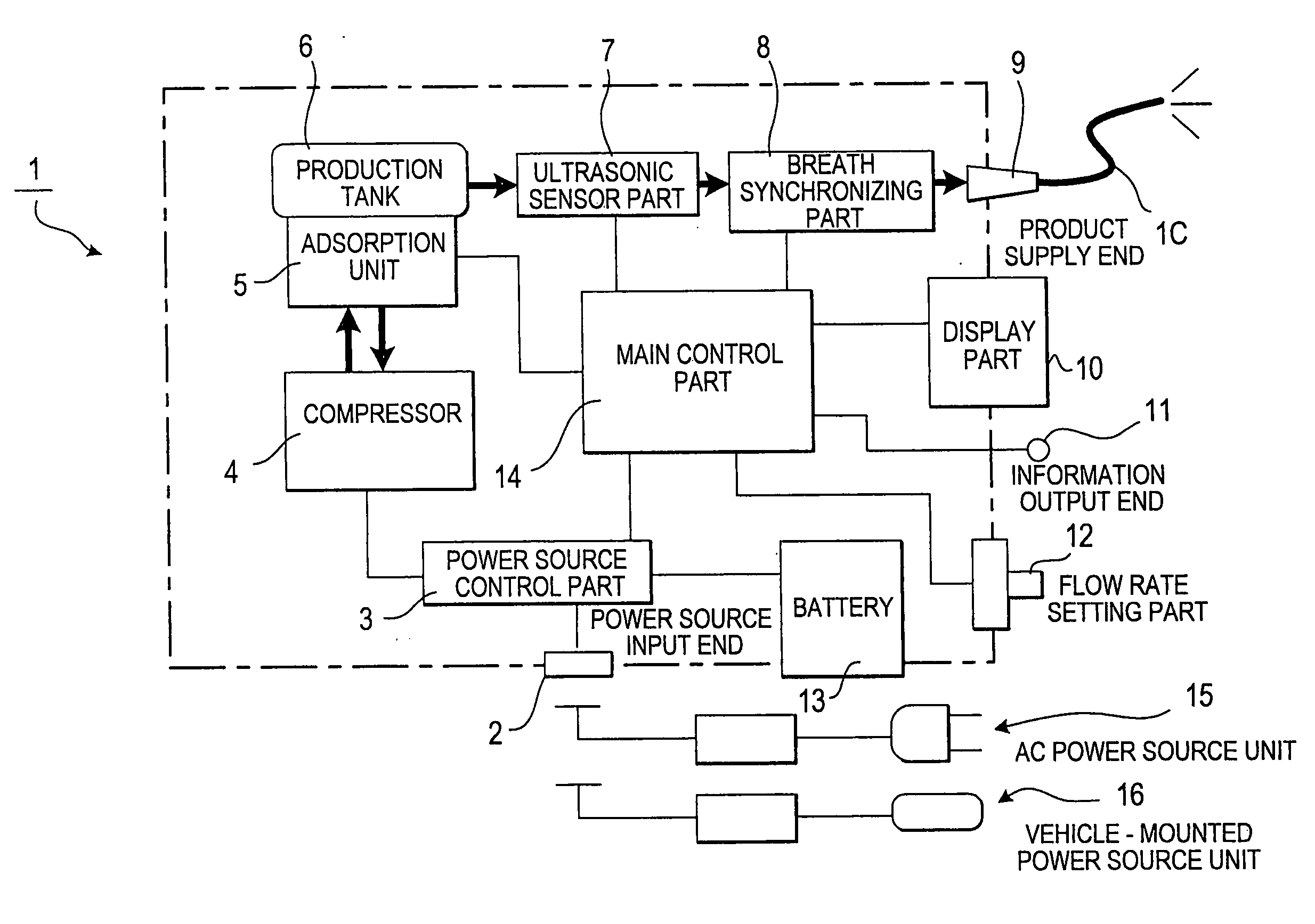
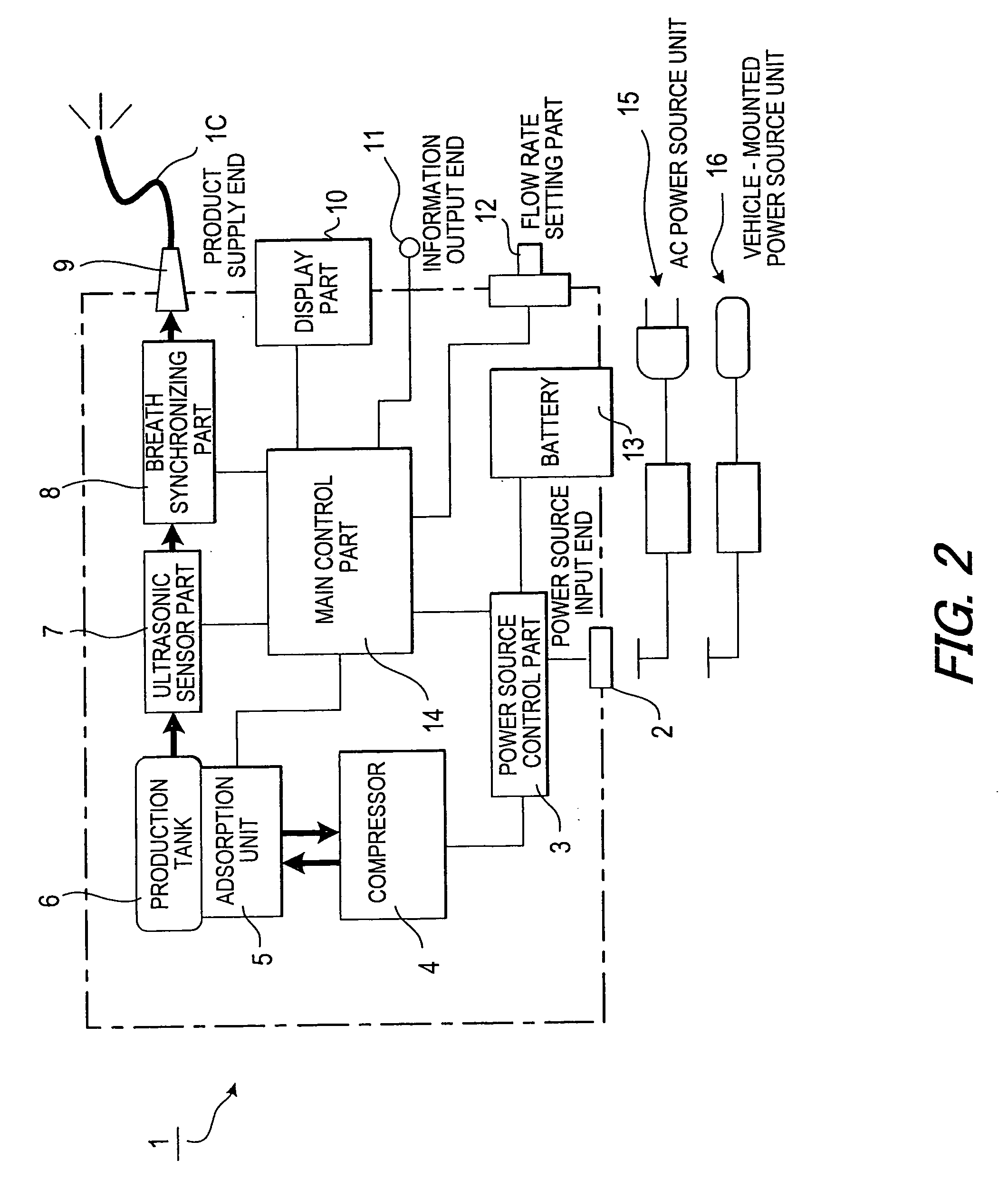
Oxygen concentrating apparatus and execution support method of home oxygen therapy using the same
ActiveUS20090281445A1Remove the burdenEliminate transmissionRespiratorsData processing applicationsInhalationHome oxygen therapy
In order to enable a medical worker to certainly and easily know whether a patient on a home oxygen therapy, who continues to inhale an oxygen-enriched gas at home, performs the inhalation as prescribed, a history of a supply condition of the oxygen-enriched gas supplied to the patient is recorded and held as supply history information, this supply history information is compared with a prescription of the oxygen therapy of the patient to generate patient's compliance information to indicate the degree to which the oxygen therapy is performed in accordance with the prescription, the oxygen concentrating apparatus is constructed to be portable, and a doctor can confirm the patient's compliance information at the time of going to a medical institution regularly.
Owner:TEIJIN LTD
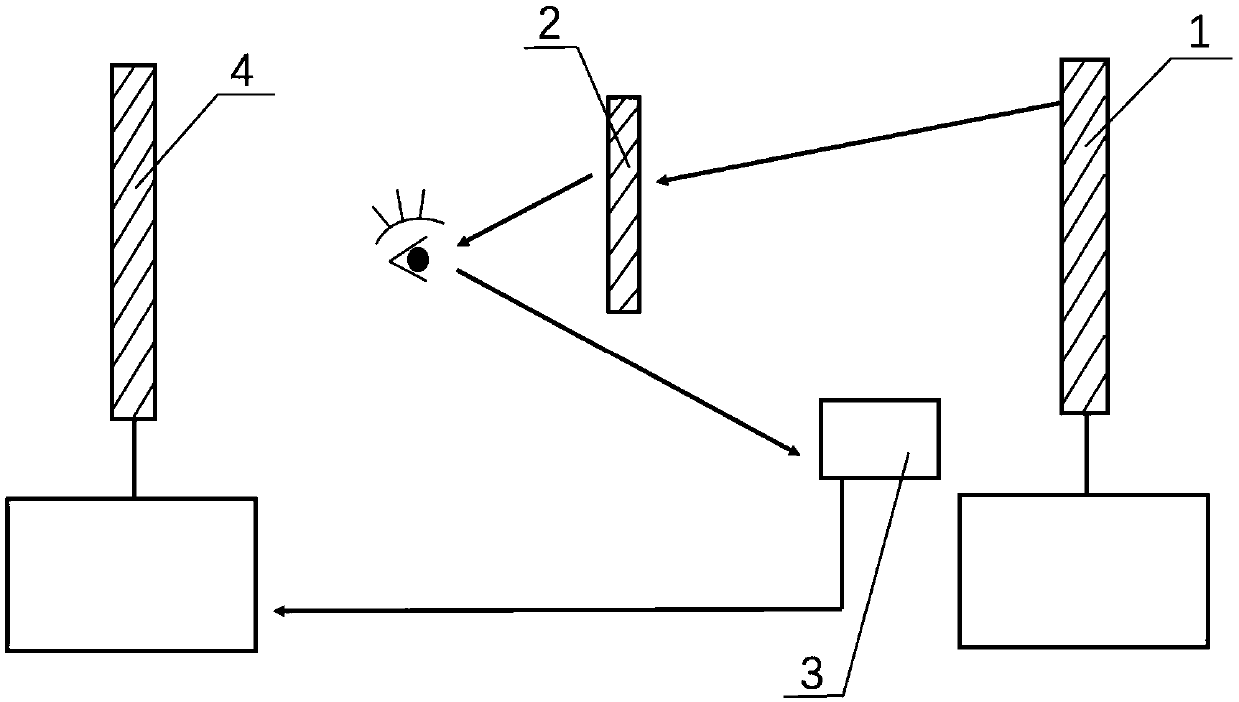
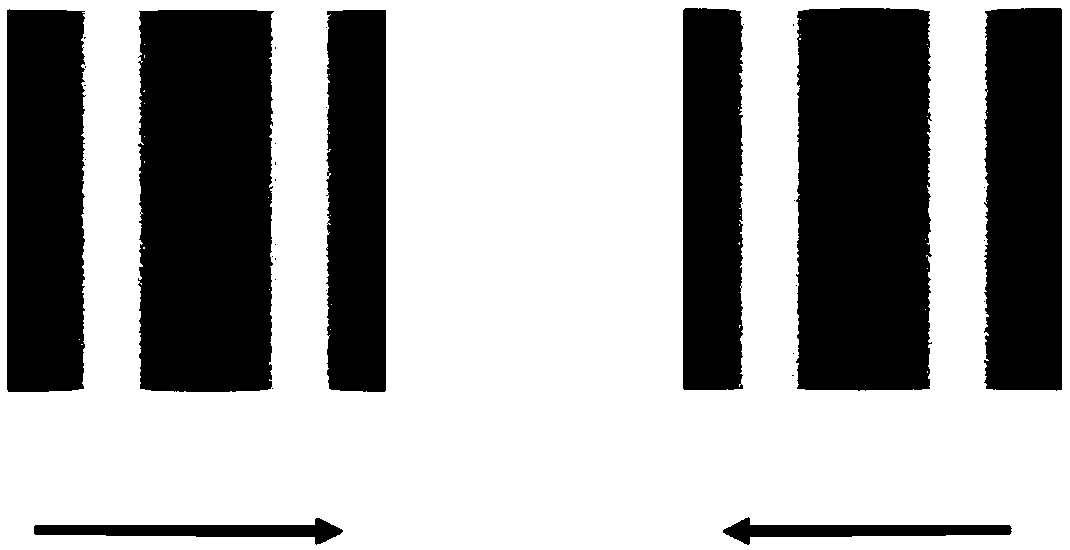
Setting system for objectively quantizing binocular inhibition degree of groups
The invention relates to the technical field of visual cognition, in particular to a setting system and method for objectively quantizing binocular inhibition degree of groups. A screen of a tested machine in the setting system displays visual stimulation, black and white sine wave bar screens which move oppositely and have different contrast proportions induce tested optokinetic nystagmus (OKN),the OKN is shown in two eyes separately by a sight glass to induce binocular competition, an eye tracker objectively records eyeball movement conditions under different contrast proportion conditionsand displays the eyeball movement conditions on a host tester screen in real time, and a binocular competition balance point is calculated by analyzing eye movement recorded data. The setting system can objectively and quantitatively evaluate the binocular inhibition degree of groups, solves the problem that the prior art cannot be applied to tests with poor adaptability or make subjective judgments, improves the evaluation accuracy of inhibition degree and relieves complete dependence of existing methods on group compliance.
Owner:EYE & ENT HOSPITAL SHANGHAI MEDICAL SCHOOL FUDAN UNIV
Paired/matched medication
This patent pairs / matches medication components. This may help with more accurate dosing when the need arises to split tablets. Also there may be increased patient compliance with patients taking many medications. Additionally, this patent would function competition to combination patents, thus driving down the cost for consumers.
Owner:SUNDHARADAS RENJIT
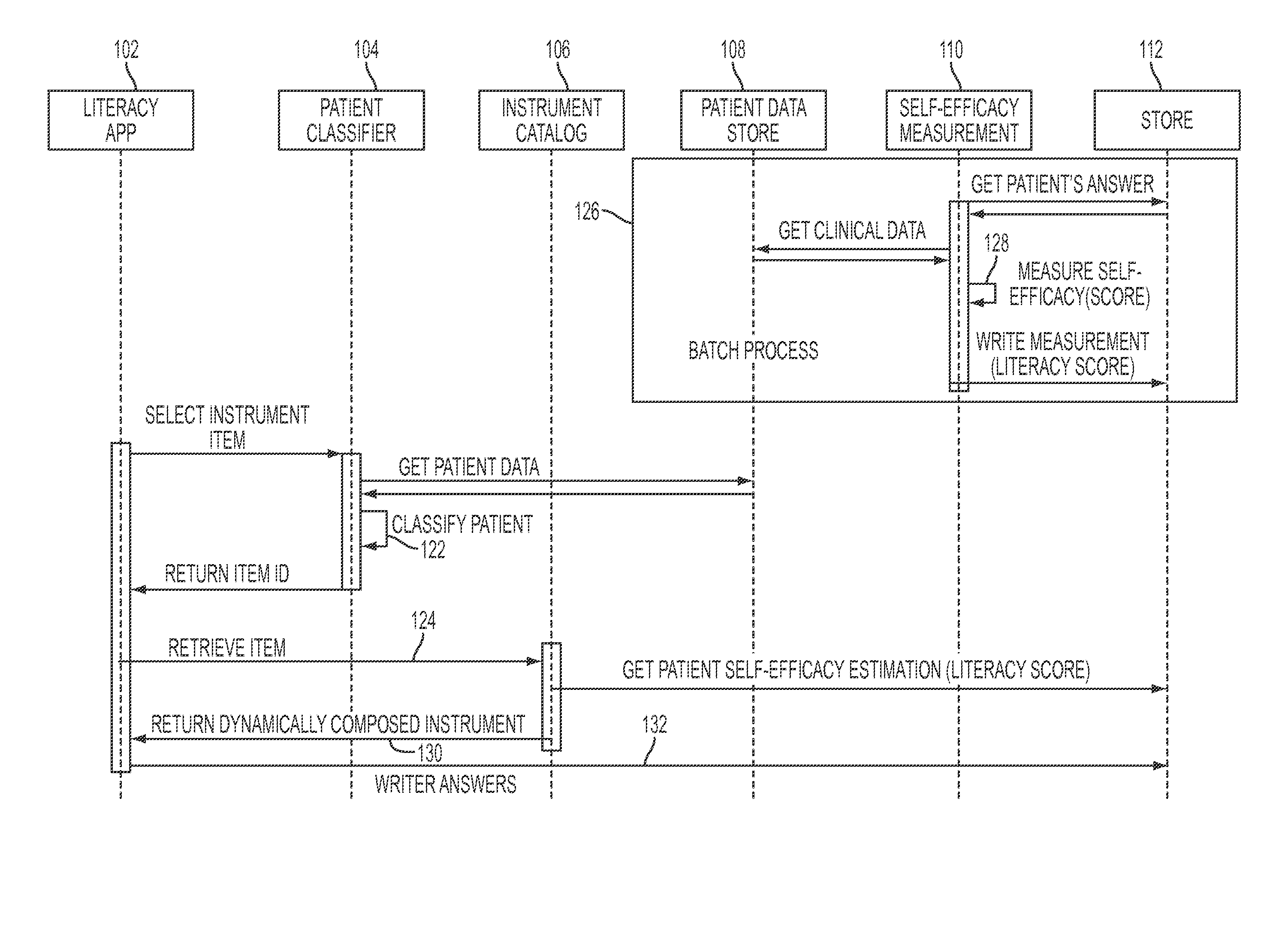
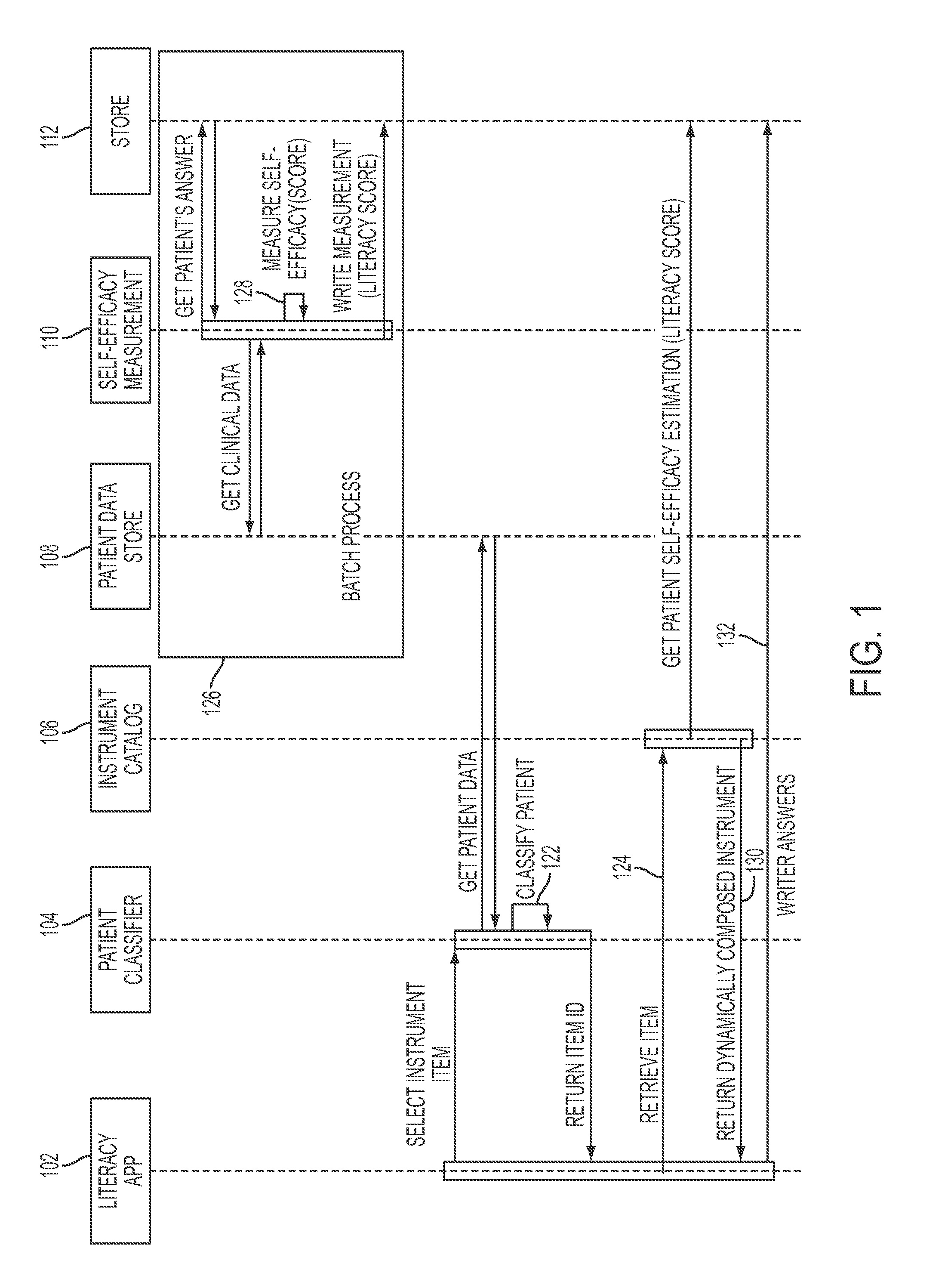
Dynamic and accretive composition of patient engagement instruments for personalized plan generation
ActiveUS20160342771A1Physical therapies and activitiesComputer-assisted medical data acquisitionPersonalizationPoint of care
Instruments of determined level of self-efficacy may be chosen dynamically for customized patient engagement, e.g., based on a patient's latent adherence trait estimated from lifestyle and other clinical data. Customization points in care plans may be identified, e.g., by monitoring an accumulative change in the patient's health literacy level. Clinical decision support at the point of care may be provided by adjusting patient engagement strategies and allocating resources accordingly.
Owner:IBM CORP
Chewable eye health formulation
ActiveUS9950008B1Hydroxy compound active ingredientsInorganic active ingredientsPoor adherenceCvd risk
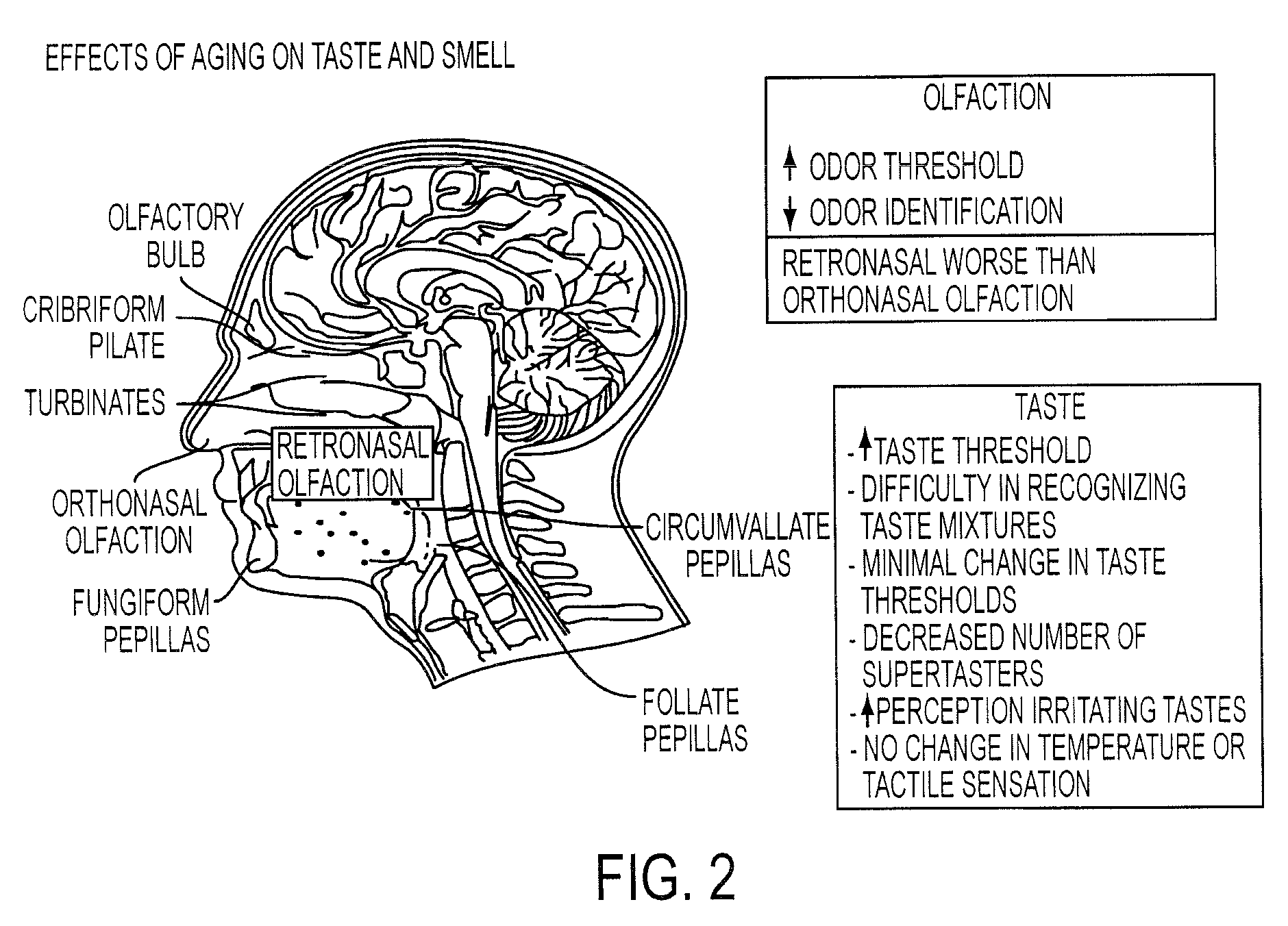
Risk of visual impairment including Age-Related Macular Degeneration (AMD) and cataracts increase with age. This increased risk can be caused by changes in the structure and function of the eye or changes in structures of the central nervous system that support visual perception. The present invention comprises fruit flavored, chewable formulations of supplements for reducing the risk of AMD. Chewable dosage forms of the invention are preferred over other formulations and may increase compliance in an aging population, who are more likely to require an eye health supplement and have an increased risk of experiencing dysphagia.
Owner:JAMIESON LAB
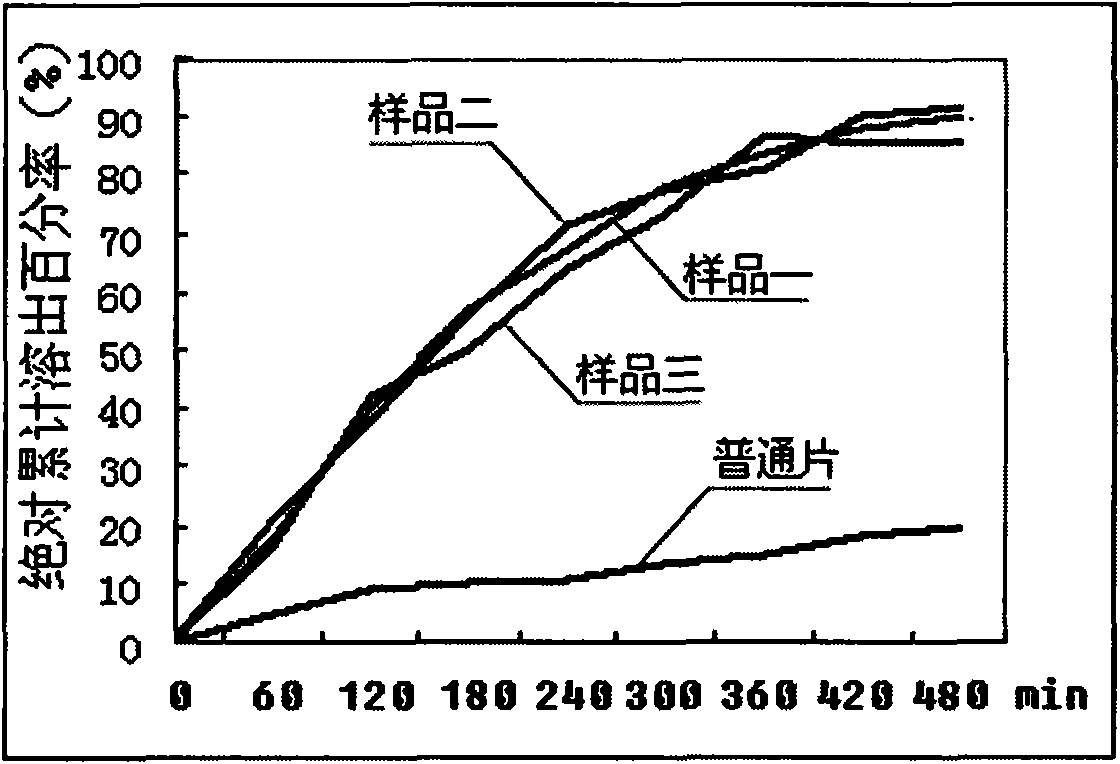
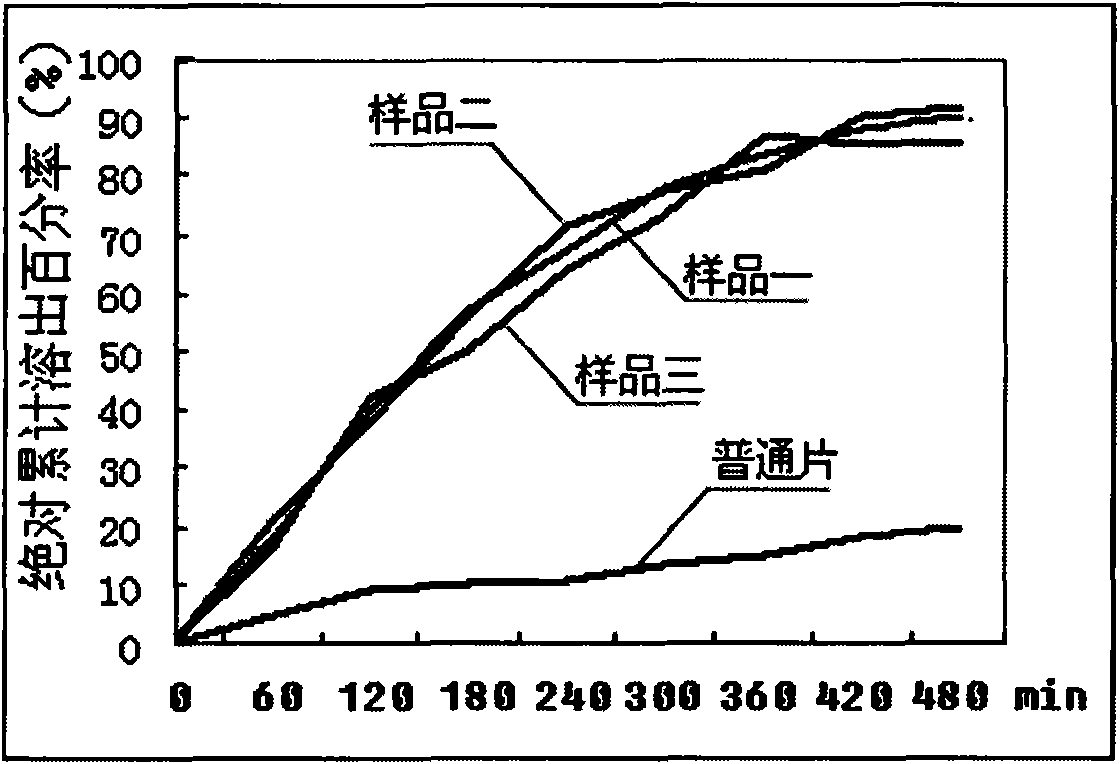
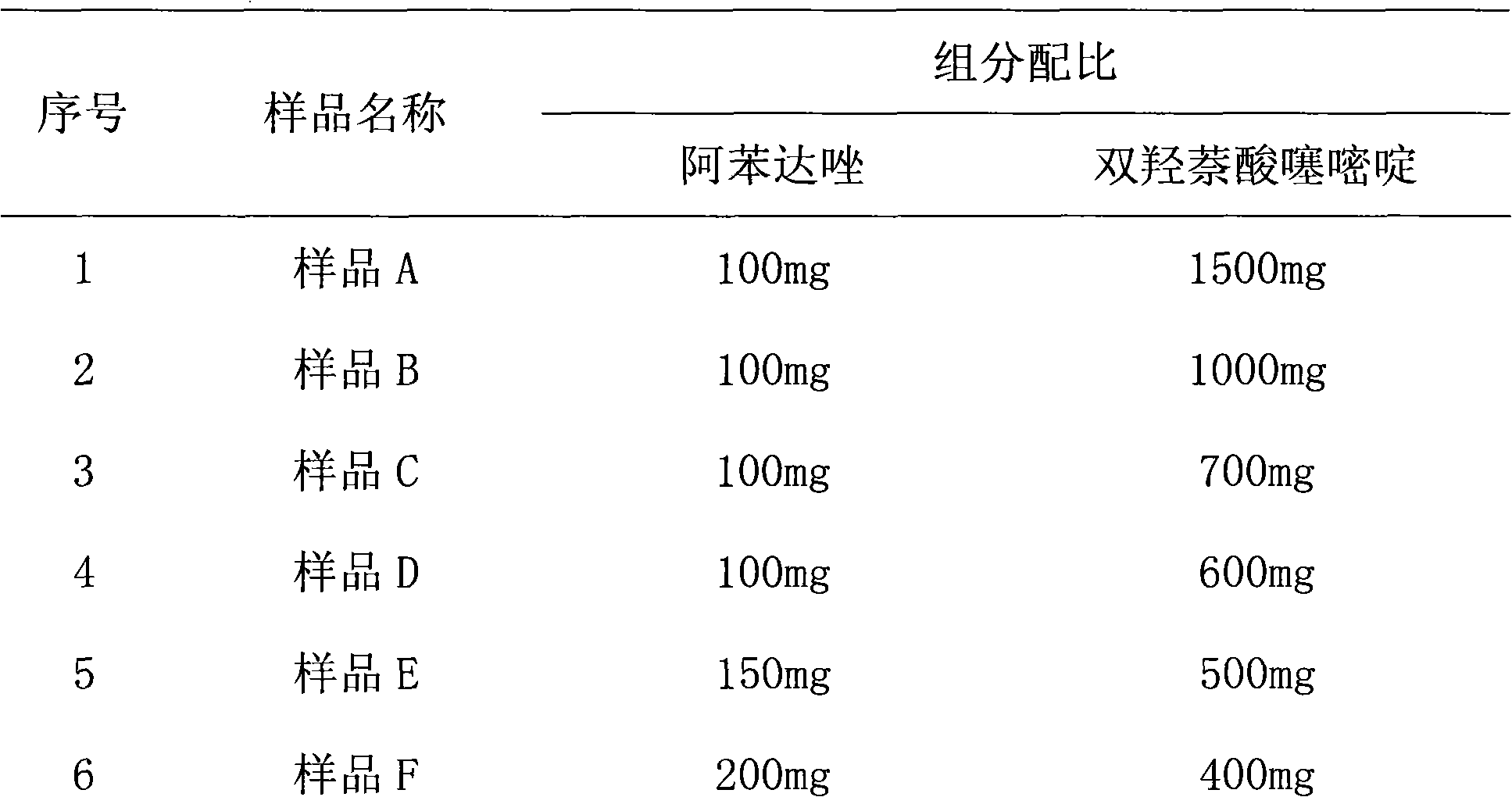
Novel dosage form containing albendazole and application and preparation method thereof
InactiveCN101919856AGood dispersionShort disintegration timeOrganic active ingredientsAntiparasitic agentsPoor adherenceSusceptible population
The invention discloses a novel dosage form containing albendazole and application and a preparation method thereof. The novel dosage form mainly takes the mixture of albendazole and pamoic acid pyrantel as a medicinal composition, and an excipient and a flavoring agent are added to the medicinal composition to be made into granules. The novel dosage form contains 500-800 parts of medicinal composition, 40-120 parts of excipient and 50-100 parts of flavoring agent in parts by weight. The granules have the advantages of good drug disperse states, short disintegration time, rapid drug dissolution, rapid absorption, high bioavailability and the like and are convenient to take. The drug mouthfeel can be regulated according to the fondness of children, such as the apple taste, the lemon taste and the like. The novel dosage form is especially suitable for children, patients with special conditions and patients who are difficult to swallow. However, the children are parasitic disease susceptible populations but have poorer drug compliance. In order to increase the drug compliance of the children and improve the drug mouthfeel and the taking mode, designing the dosage form into the granules is extremely necessary. The invention also makes the orally taken dosage form of the compound albendazole granules diversified and simultaneously provides more selections for clinician and patient medications.
Owner:SICHUAN BAOSHENGKANG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com