Roflumilast inhalation aerosol compound and preparation method thereof
A technology for roflumilast and aerosol, which is applied in the field of compound roflumilast inhalation aerosol and its preparation, can solve problems such as complicated devices, and achieve the effects of eliminating damage, improving lung deposition, and improving compliance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1. Formula (calculated based on 1000 bottles):
[0049] Roflumilast: 24.4g;
[0050] Salbutamol: 12.2g;
[0051] Lecithin: 4.8g;
[0052] Absolute ethanol: 1200g;
[0053] HFA: added up to 18000g.
[0054] 2. Preparation method:
[0055] 1) Micronization of API: pulverize albuterol sulfate to less than 7 μm;
[0056] 2) Weighing: Weigh the roflumilast, albuterol, lecithin, and absolute ethanol in the formula amount;
[0057] 3) Surfactant dissolution: first take 2 / 3 of the formula amount of absolute ethanol, add the formula amount of lecithin, and homogenize and stir for 20 minutes under a high-shear homogenizer;
[0058] 4) Preparation of medicinal solution: add the upper liquid to the formulated amount of salbutamol sulfate, stir for 30 minutes under a high-shear homogenizer; supplement the remaining anhydrous ethanol to the full amount, continue stirring for 20 minutes, turn on the circulation pump, and circulate for 15 minutes;
[0059] 5) Filling and pinchin...
Embodiment 2
[0066] 1. Formula (calculated based on 1000 bottles):
[0067] Roflumilast: 28g;
[0068] Salbutamol: 14g;
[0069] Mint ice: 50g;
[0070] Propylene glycol: 350g;
[0071] HFA: added up to 14000g.
[0072] 2. Preparation method:
[0073] See Example 1.
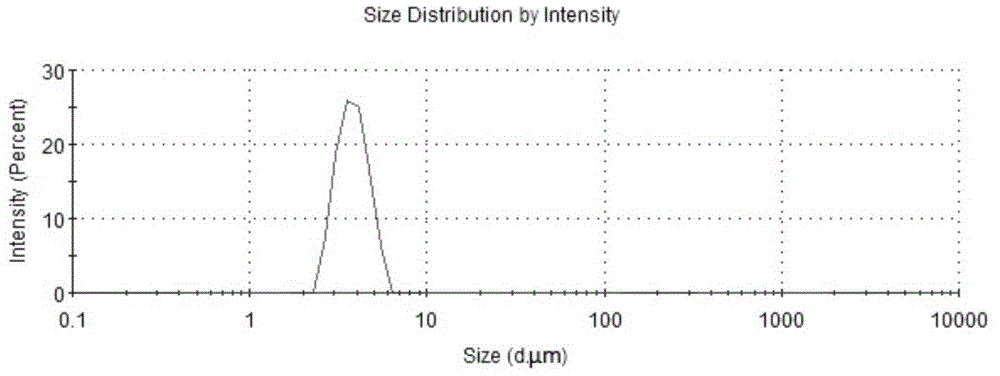
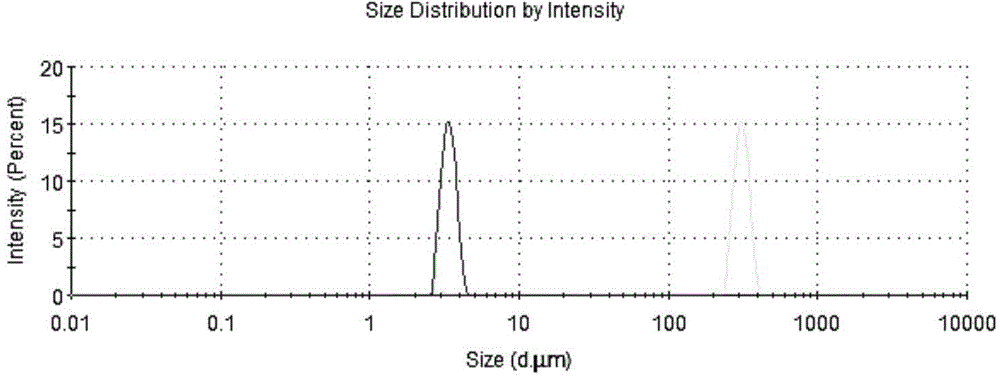
[0074] 3. Particle size determination
[0075] Particle size determination method is referring to embodiment 1, and measurement result is shown in Table 2 and figure 2 .
[0076] Table 2 Particle Size Distribution
[0077]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com