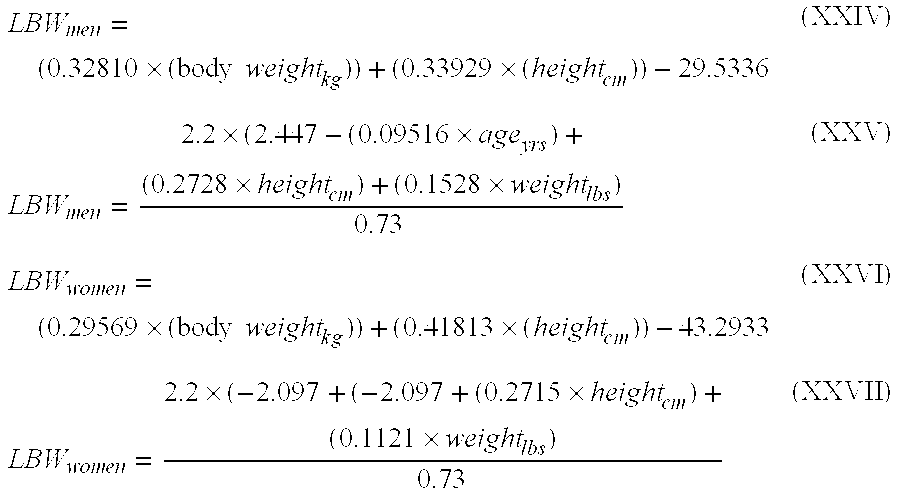Patents
Literature
247 results about "Policy Compliance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In general, compliance means conforming to a rule, such as a specification, policy, standard or law. Regulatory compliance describes the goal that organizations aspire to achieve in their efforts to ensure that they are aware of and take steps to comply with relevant laws, policies, and regulations.
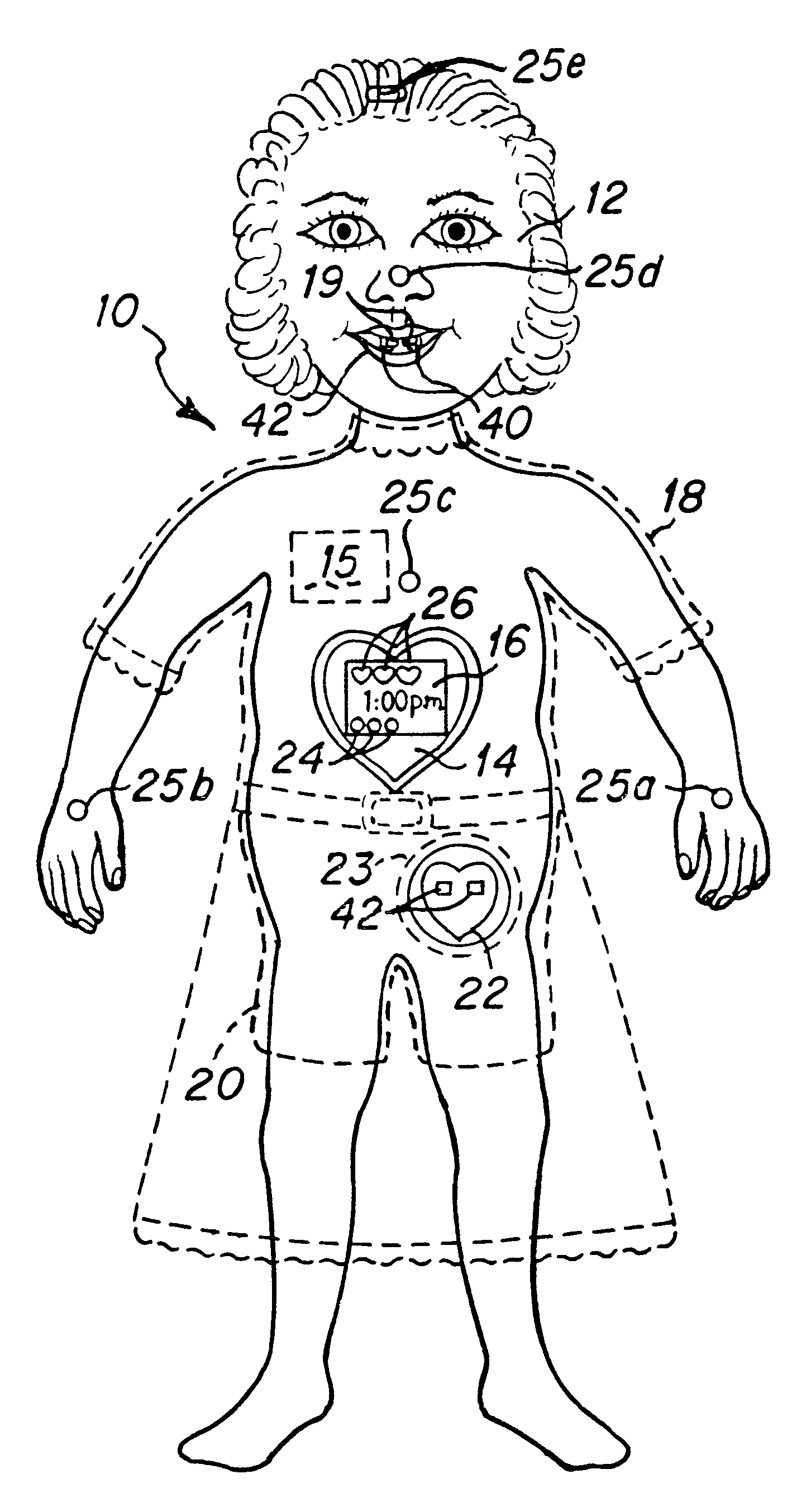
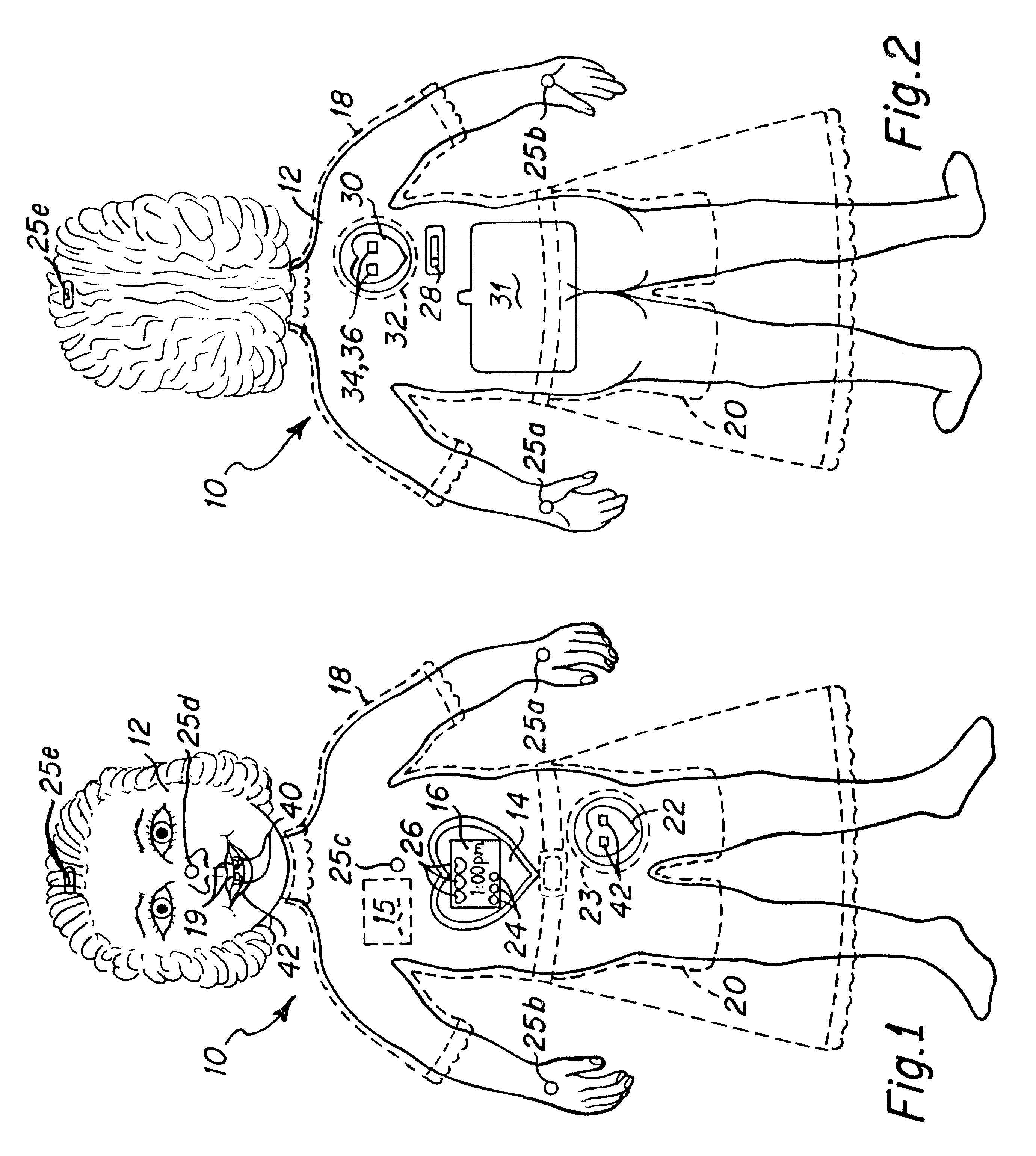
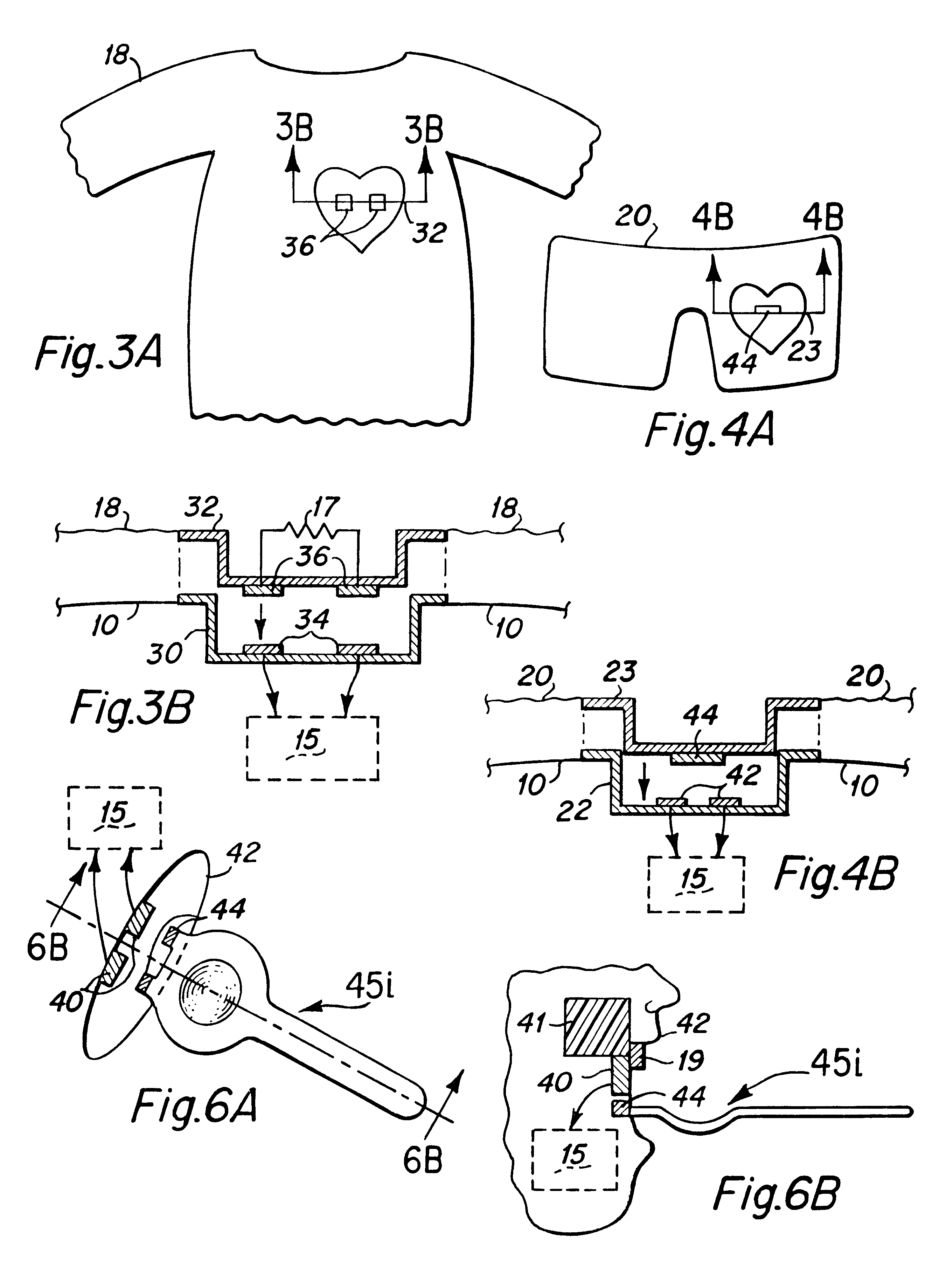
Interactive virtual character doll
The interactive doll simulates the character of a live person or, in other embodiments a fantasy figure or animal, in essence, simulating a living being that possesses respective human or animal qualities: displaying specific needs, tenderness, intelligence and / or understanding. The doll contains a clock or other timekeeping device and thereby knows of the time of day. It automatically enters a sleep mode at a preset sleep time during which the playtoy remains quiet, and wakens at a preset hour of the day, issuing a verbal statement to let the player know it is time to again play. By issuing a sequence of verbal requests from time to time to the player to take action of various kinds on or with the doll, determines the player's compliance or noncompliance with each such request and issues a verbal message appropriate to such compliance or non-compliance. Some of the verbal requests made are of a kind that occur at a particular time of day in the life of the character being synthesized, such as a request for a food or beverage at breakfast time, lunch time or supper time. And, from time to time at its own initiative, the playtoy may issue verbal messages of affection to the player. That doll is accompanied by external objects that simulate a variety of foods, a beverage, medicine and the like, which the doll is to be applied by the player to the doll pursuant to specific verbal requests from the doll and the doll is able to identify those objects for enhanced interactive play with the player.
Owner:PLAYMATES TOYS
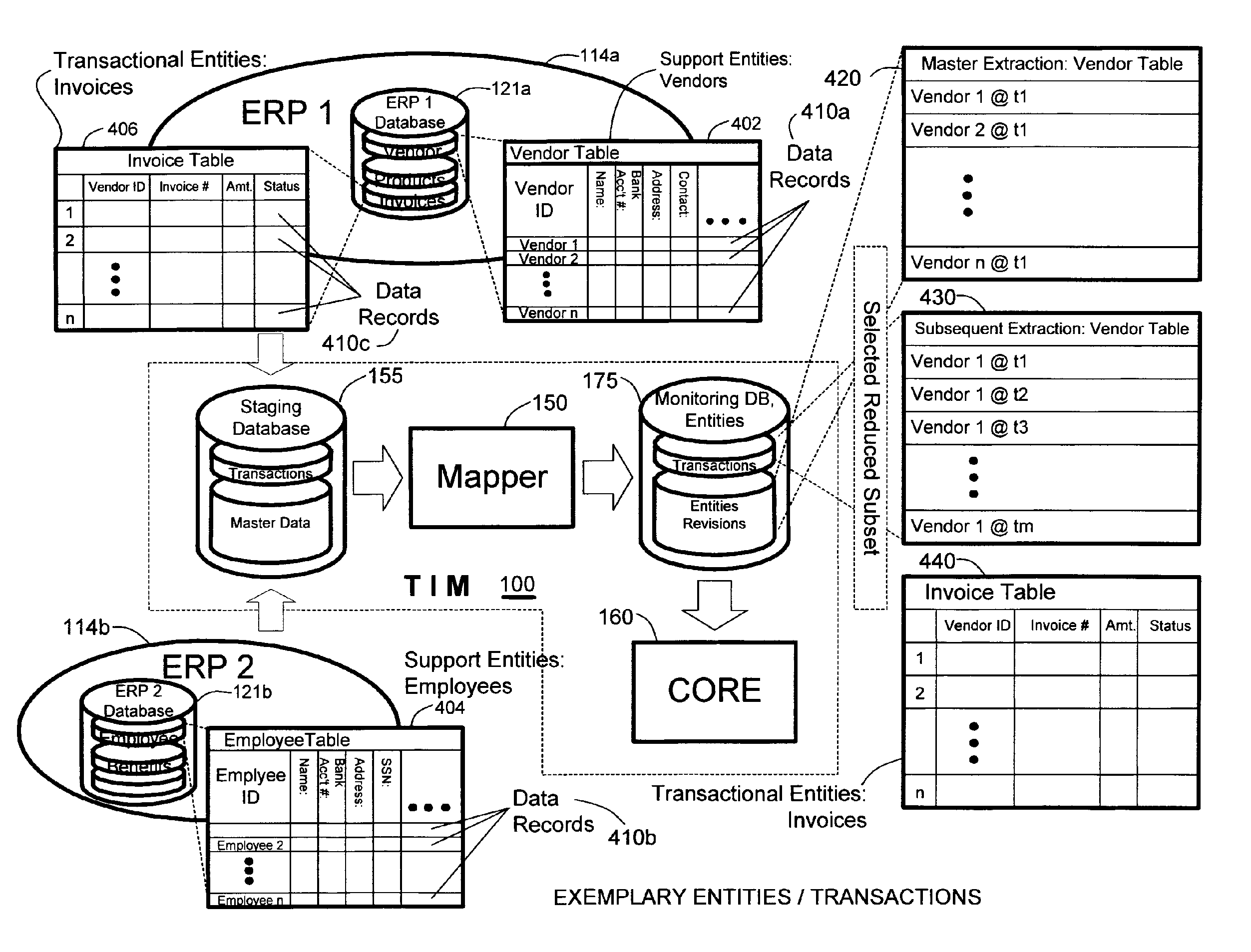
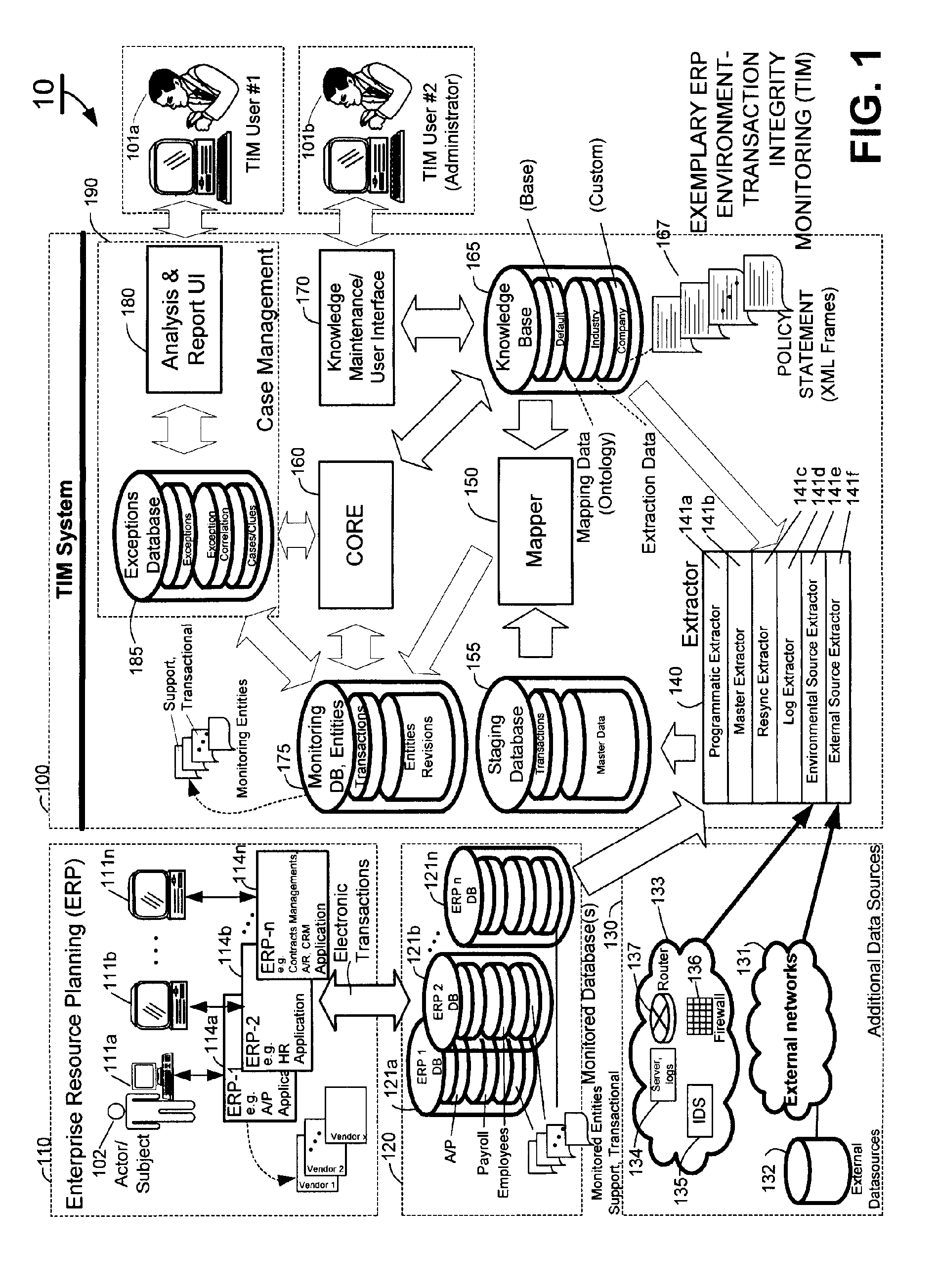
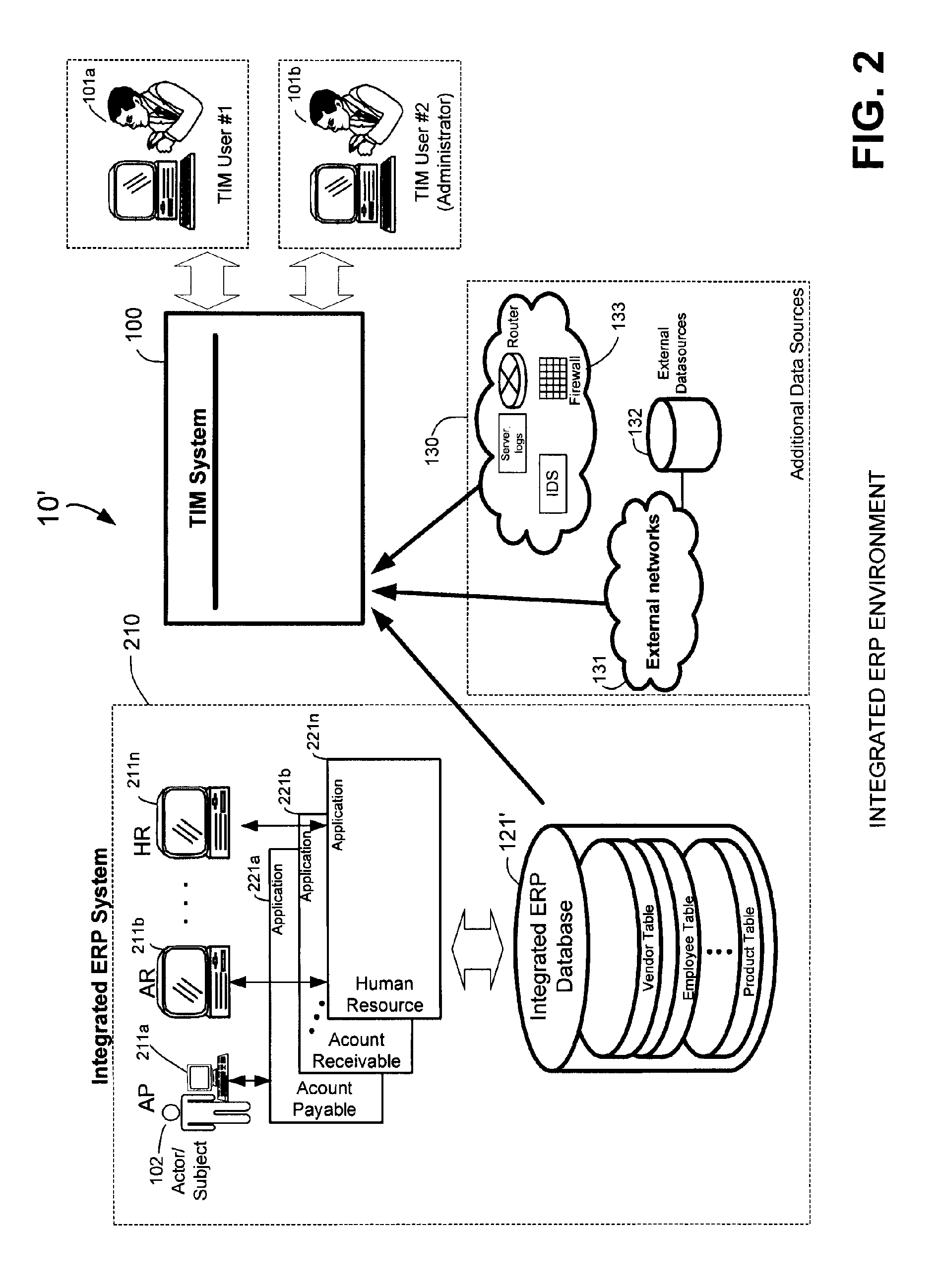
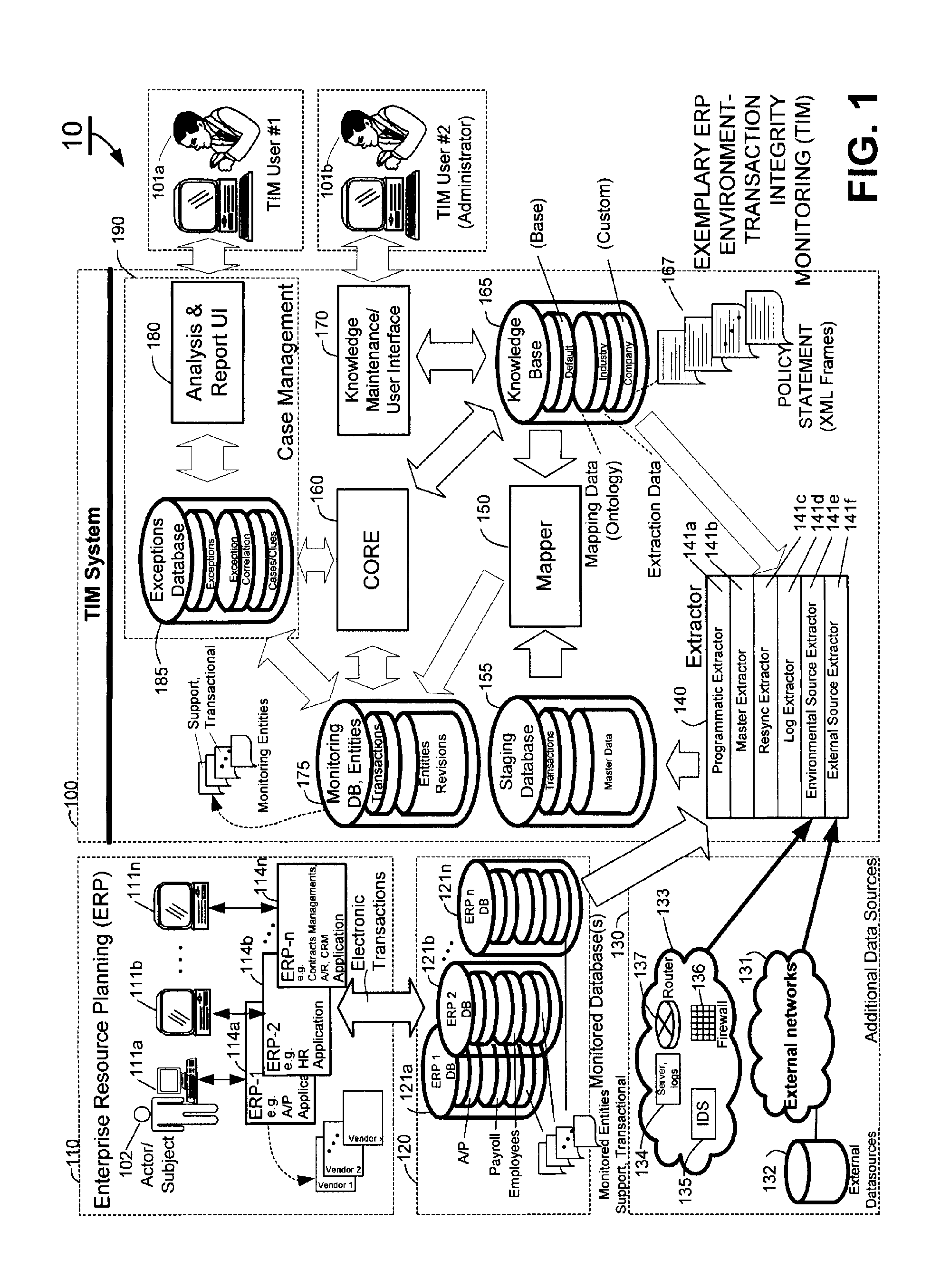
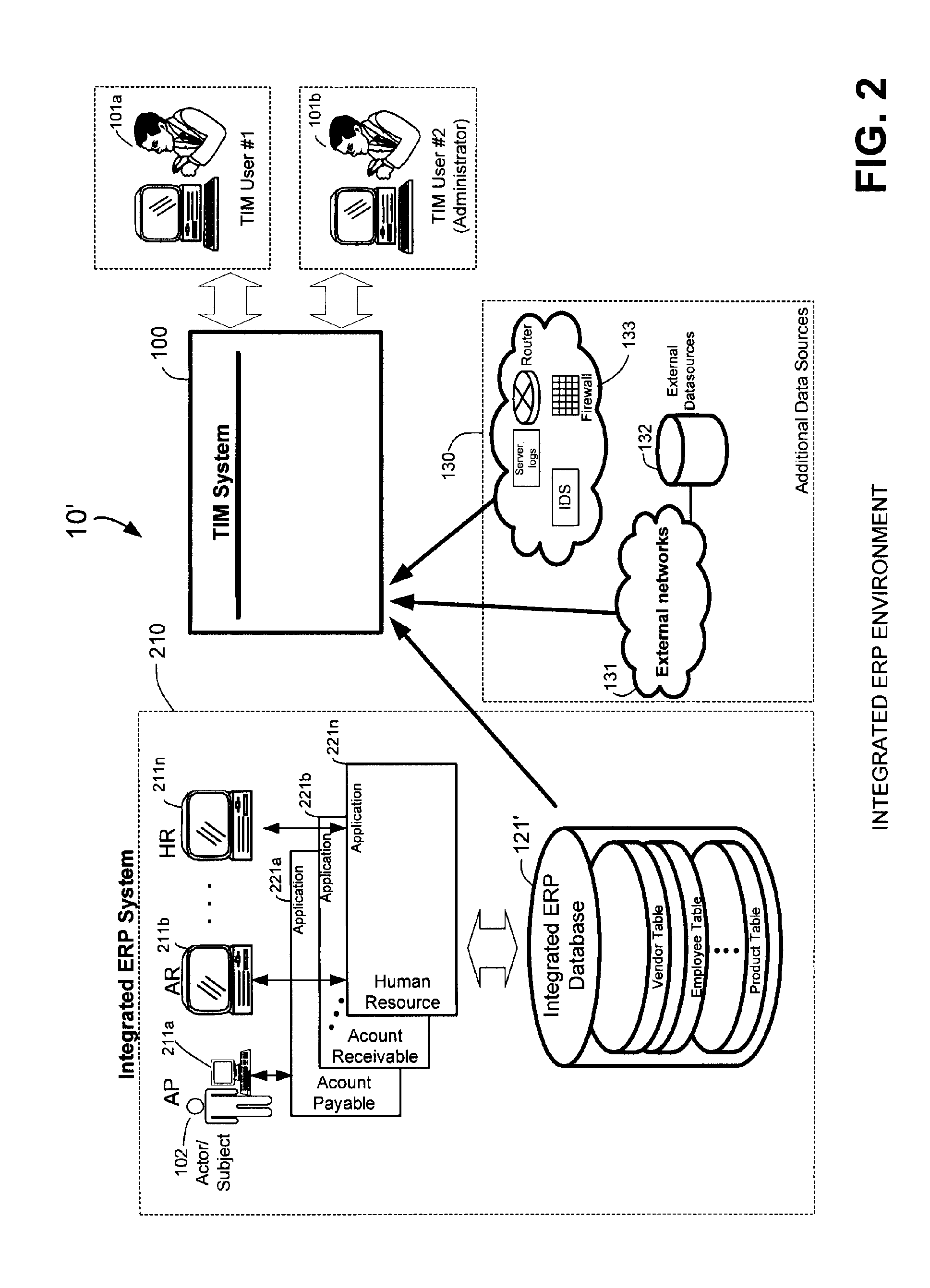
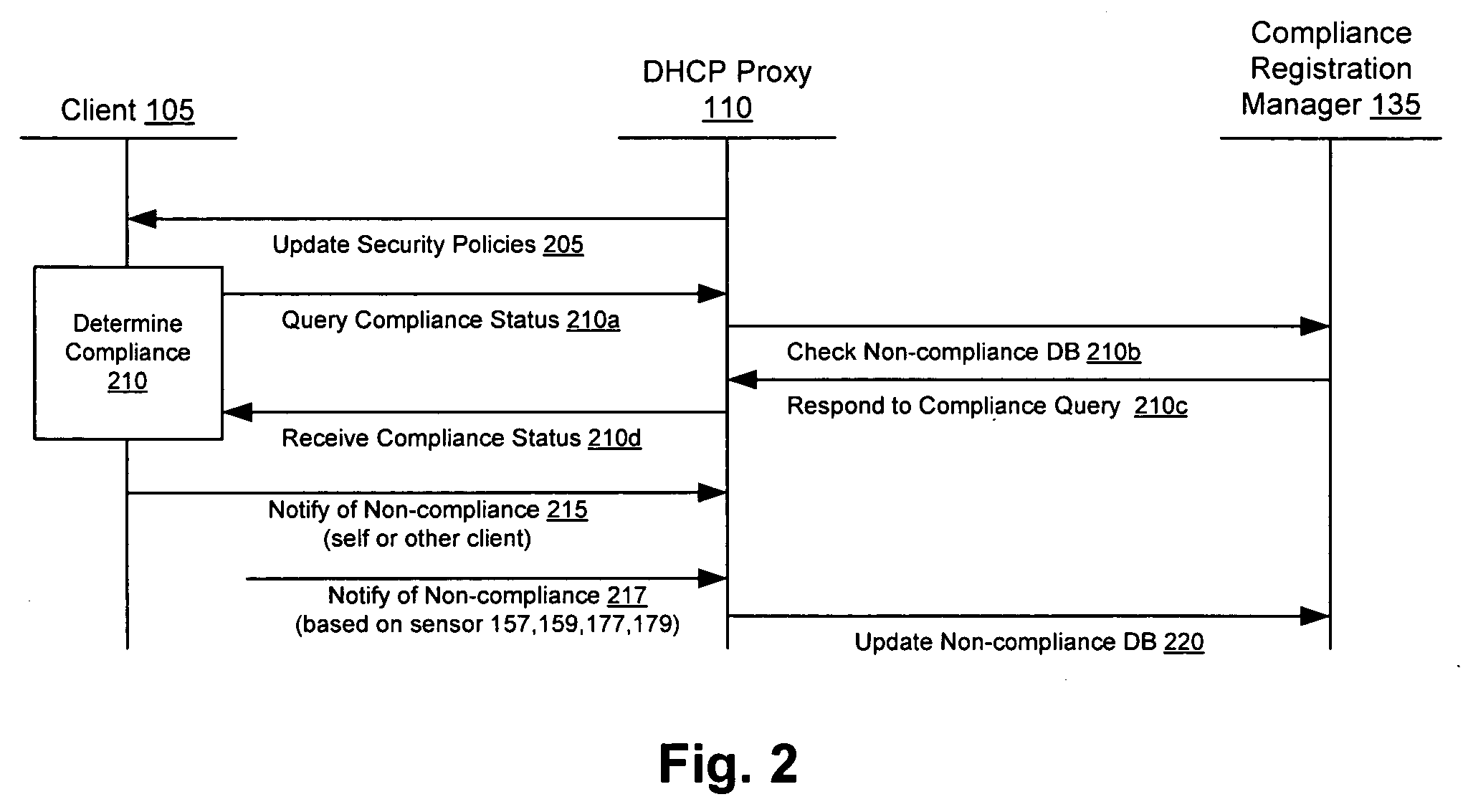
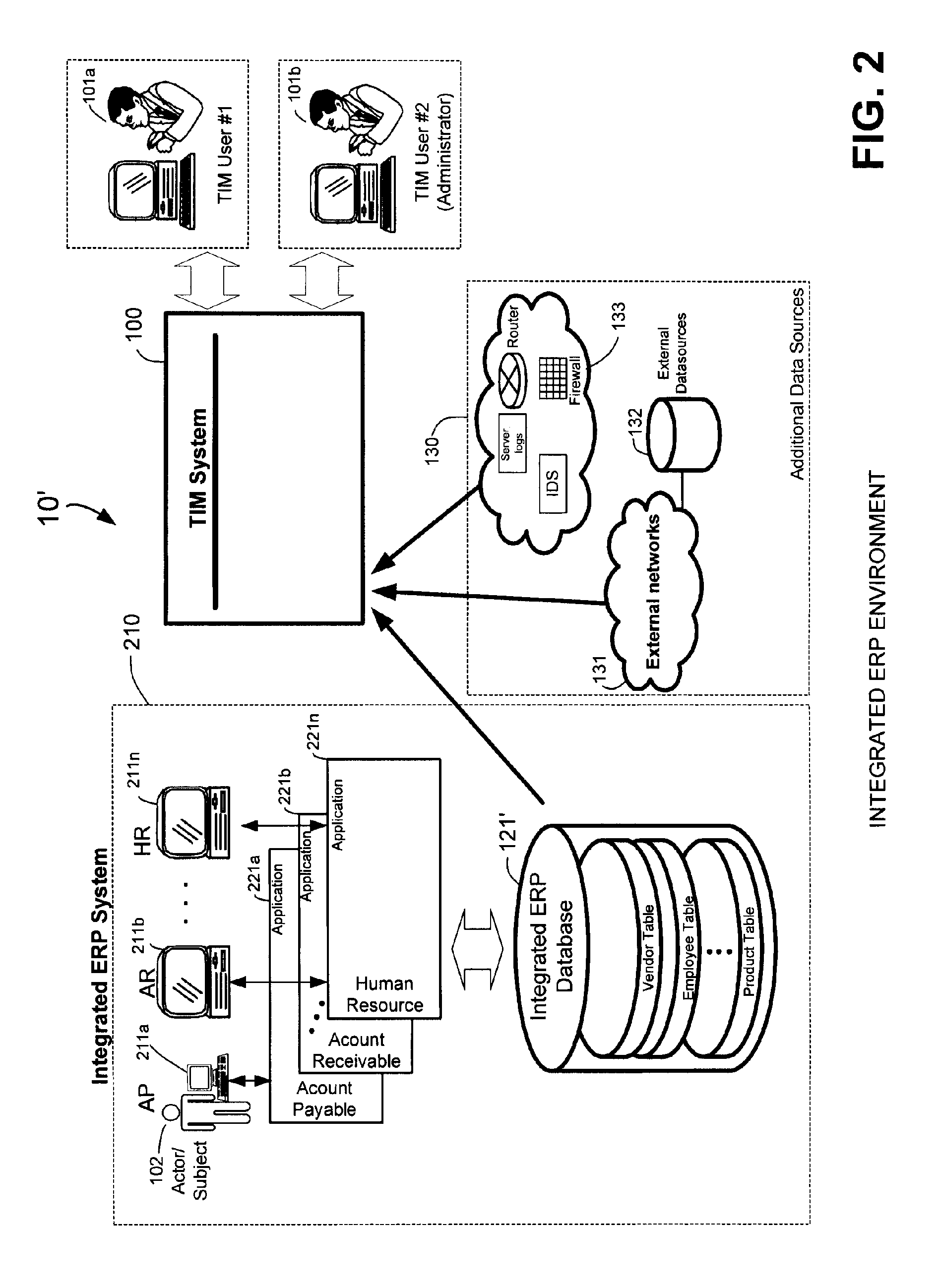
Methods and systems for compliance monitoring knowledge base
ActiveUS20060212486A1Reduced asset lossImprove operational efficiencyDigital data information retrievalFinanceCompliance MonitoringMonitoring system
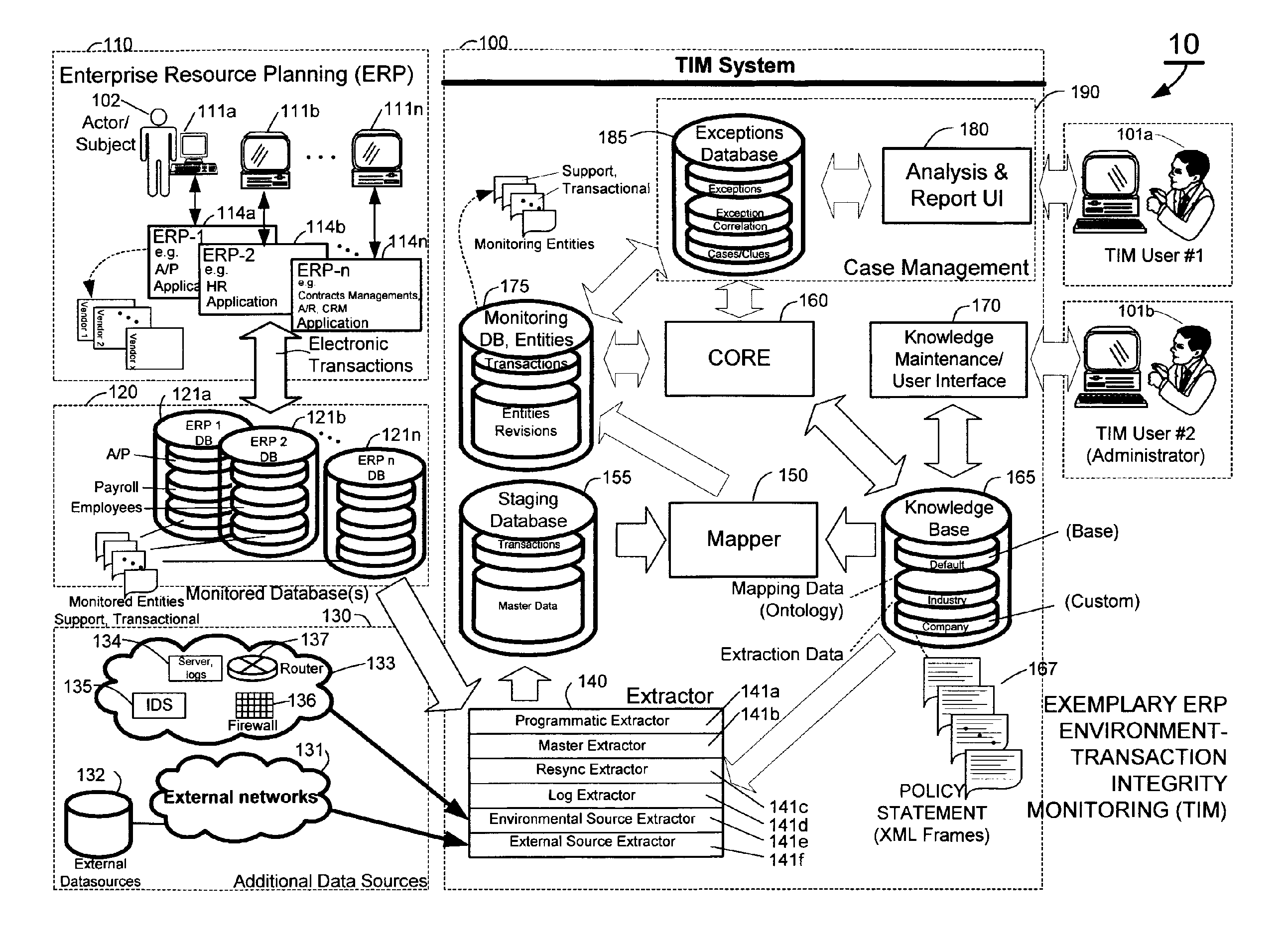
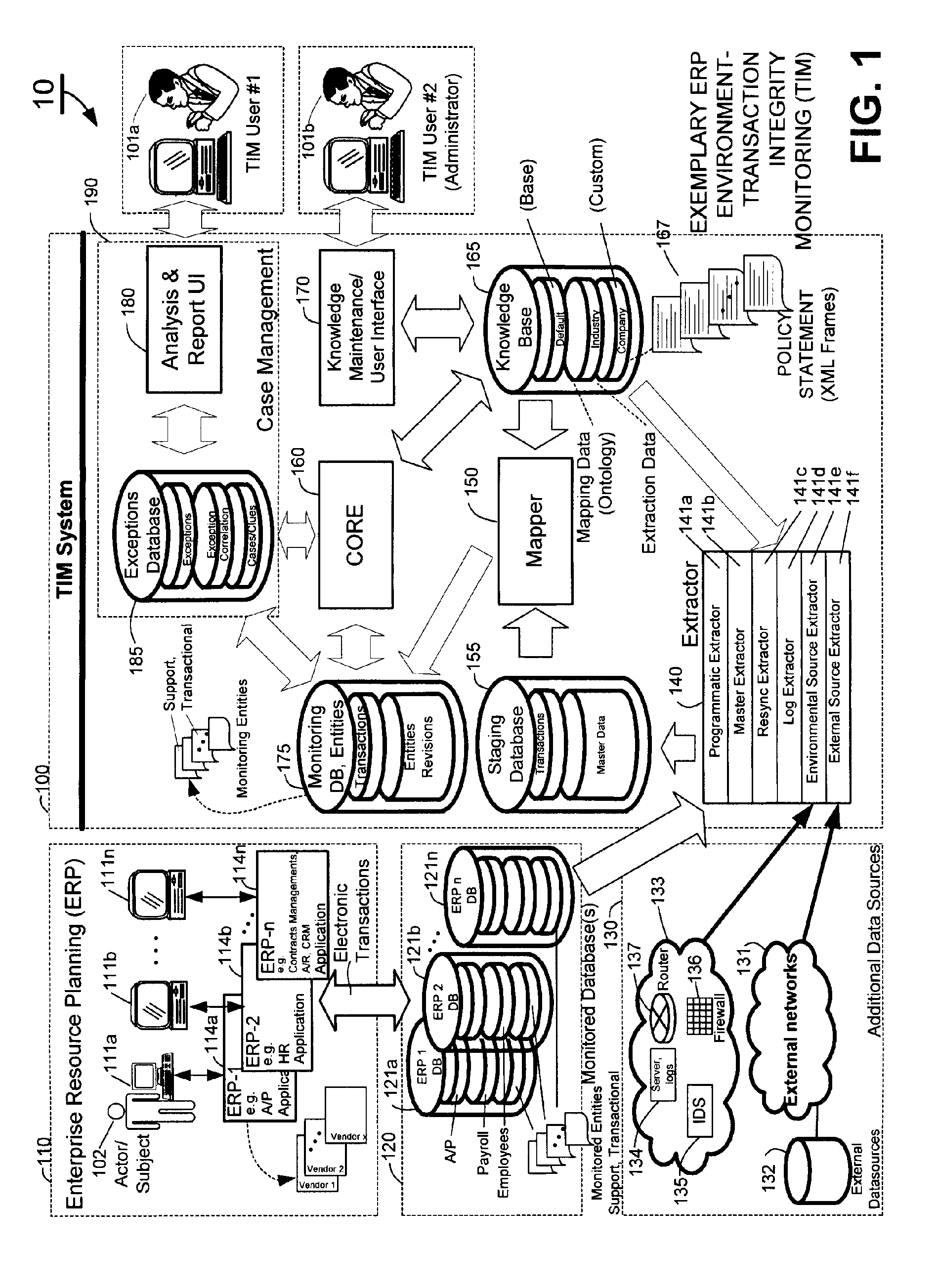
A knowledge base and methods for use in connection with a policy compliance monitoring system operative to determine exceptions to policies expressed by computer-executable policy statements. The system allows establishment, codification, and maintenance of enterprise policies, monitors electronic transactions of the enterprise from various and possibly heterogeneous data sources, detects exceptions to established policies, reports exceptions to authorized users such as managers and auditors, and / or provides a case management system for tracking exceptions and their underlying transactions. The knowledge base comprises extractor files that are utilized for extracting information from data sources for utilization in policy compliance monitoring, a mapper for normalizing data from the data sources against a system ontology and storing normalized data in a monitoring database, and computer-executable compliance policy statements used by a transaction analysis engine. The policy statements represent predetermined policies of the enterprise that apply to data stored in the monitoring database.
Owner:OVERSIGHT SYST INC
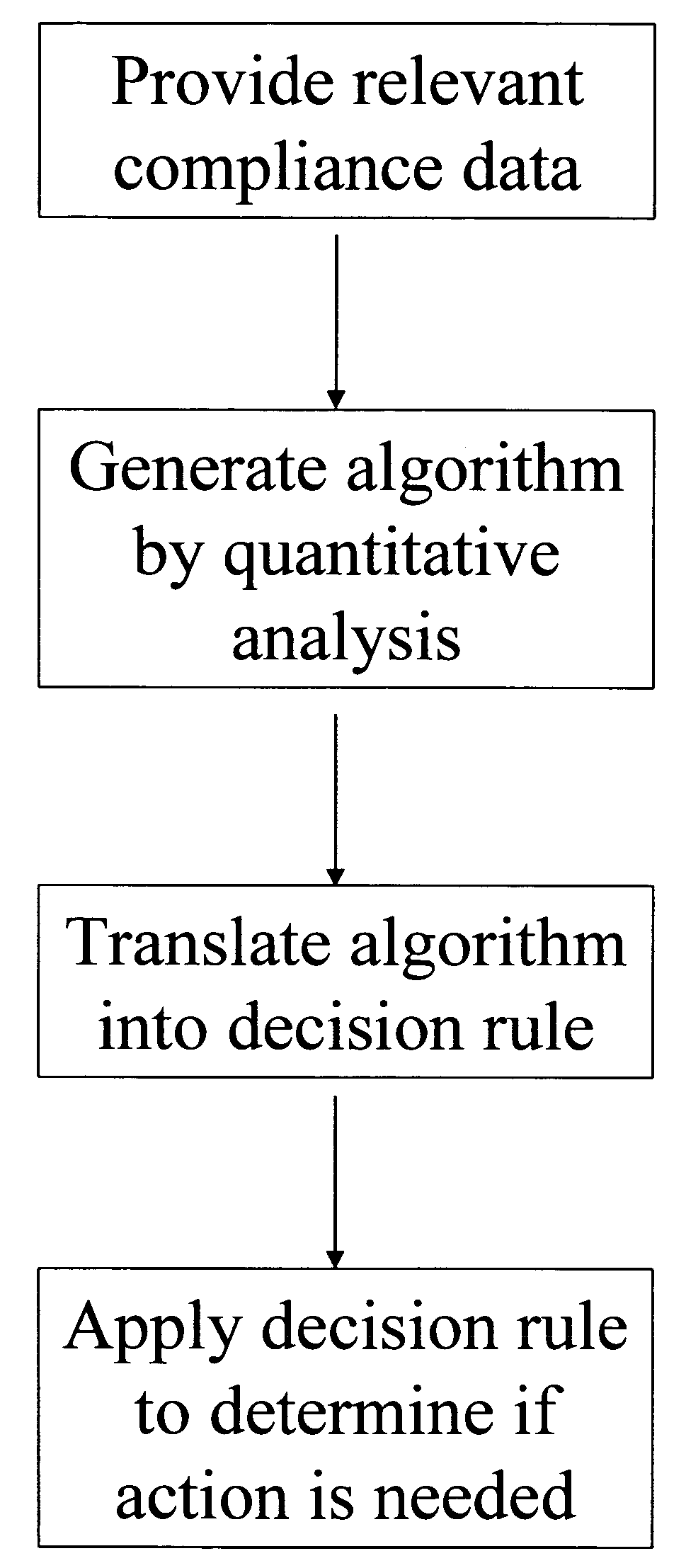
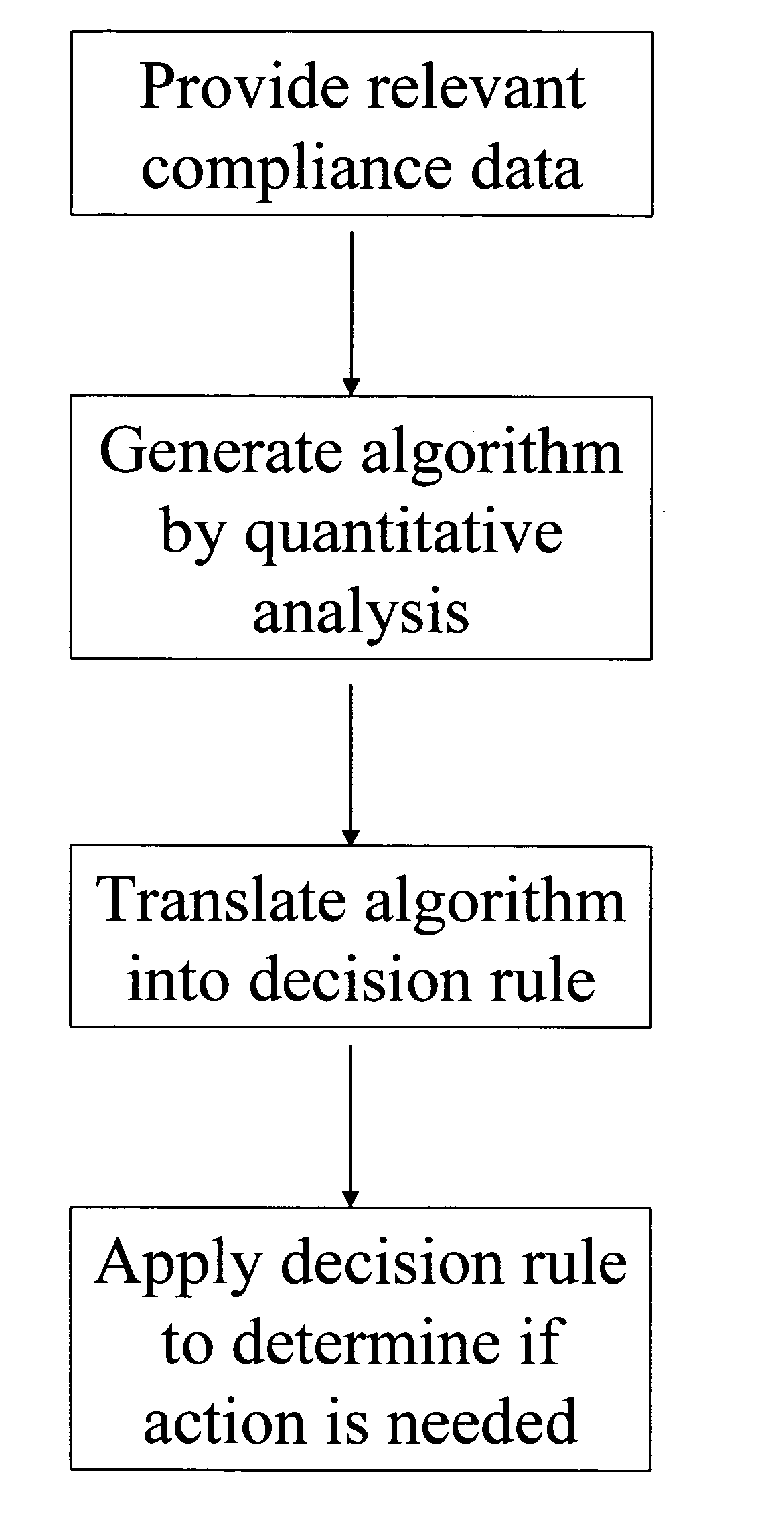
Apparatus and method for prediction and management of participant compliance in clinical research
InactiveUS7415447B2Reduce testing costsImprove statistics performanceDigital computer detailsForecastingNon complianceClinical trial
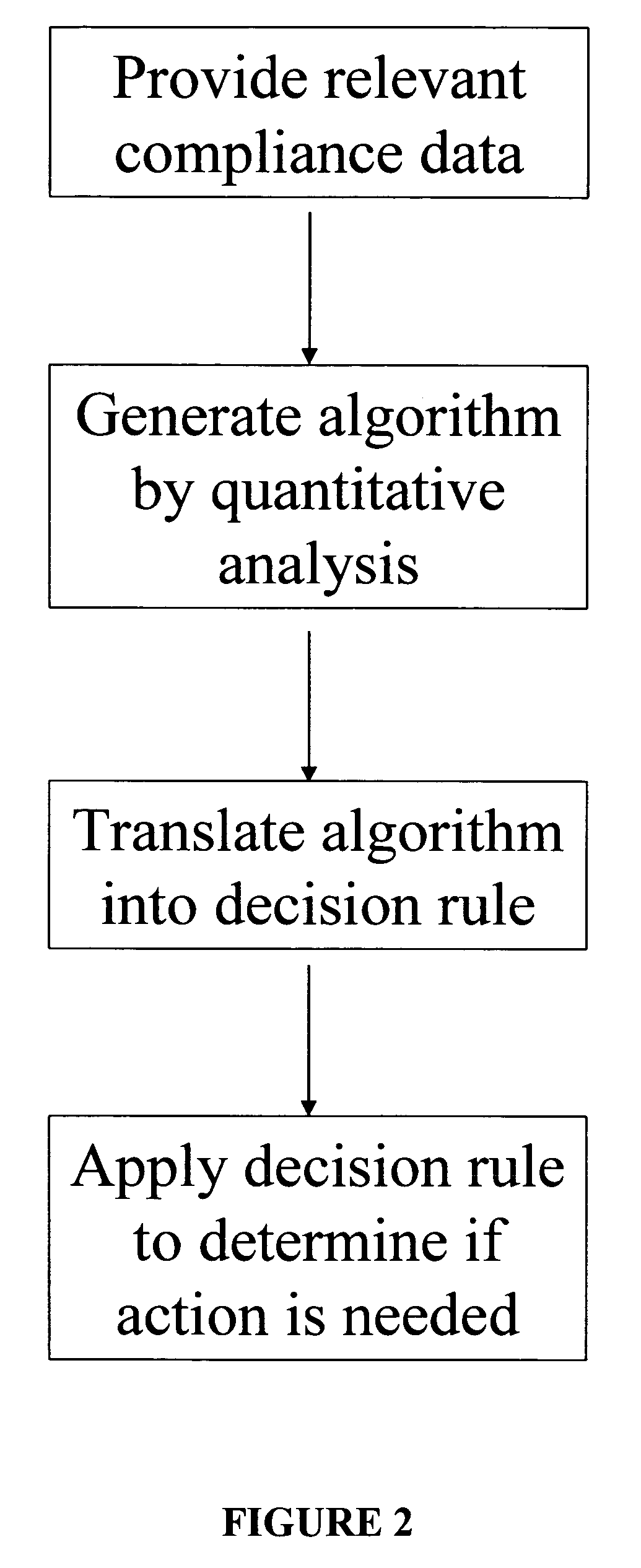
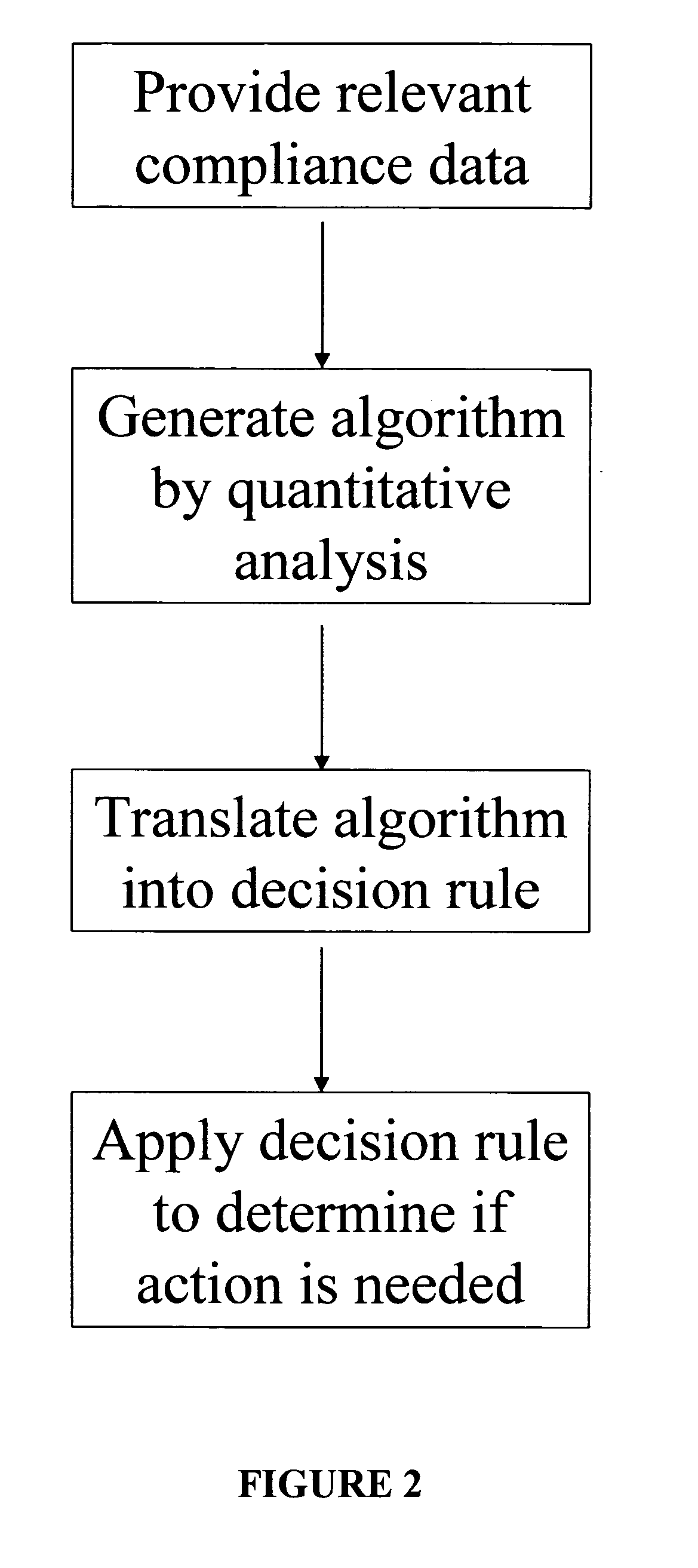
A system for developing and implementing empirically derived algorithms to generate decision rules to determine participant noncompliance and fraud with research protocols in clinical trials allows for the identification of complex patterns of variables that detect or predict participant noncompliance and fraud with research protocol, including performance and enrollment goals, in the clinical trial. The data may be used to overall predict the performance of any participant in a clinical trial, allowing selection of participants that tend to produce useful, high-quality results. The present invention can also be used to monitor participant compliance with the research protocol and goals to determine preferred actions to be performed. Optionally, the invention may provide a spectrum of noncompliance, from minor noncompliance needing only corrective feedback, to significant noncompliance requiring participant removal from the clinical trial or from future clinical trials. The algorithms and decision rules can also be domain-specific, such as detecting non-compliance or fraud among subjects in a cardiovascular drug trial, or demographically specific, such as taking into account gender, age or location, which provides for algorithms and decision rules to be optimized for the specific sample of participants being studied.
Owner:ERESTECH
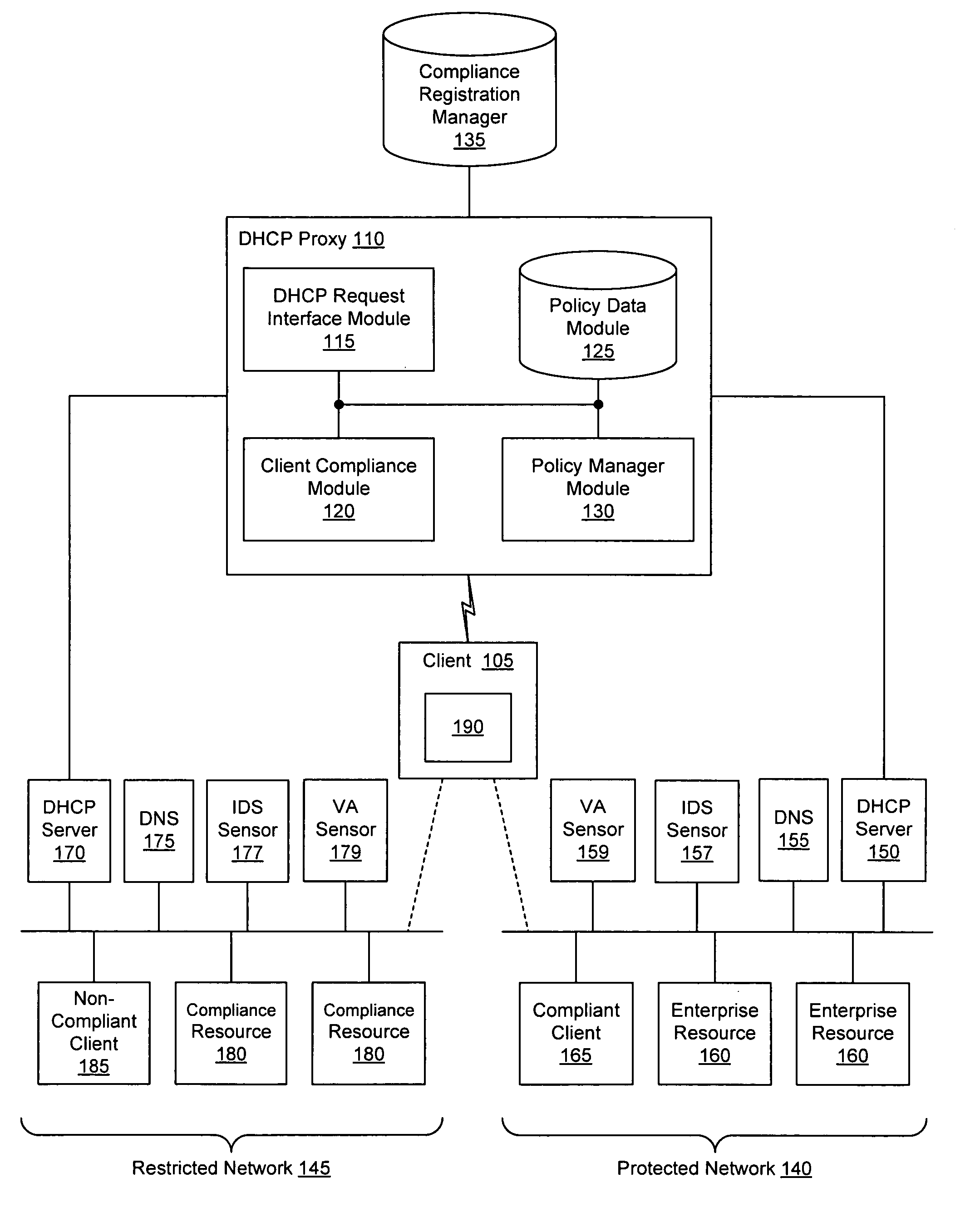
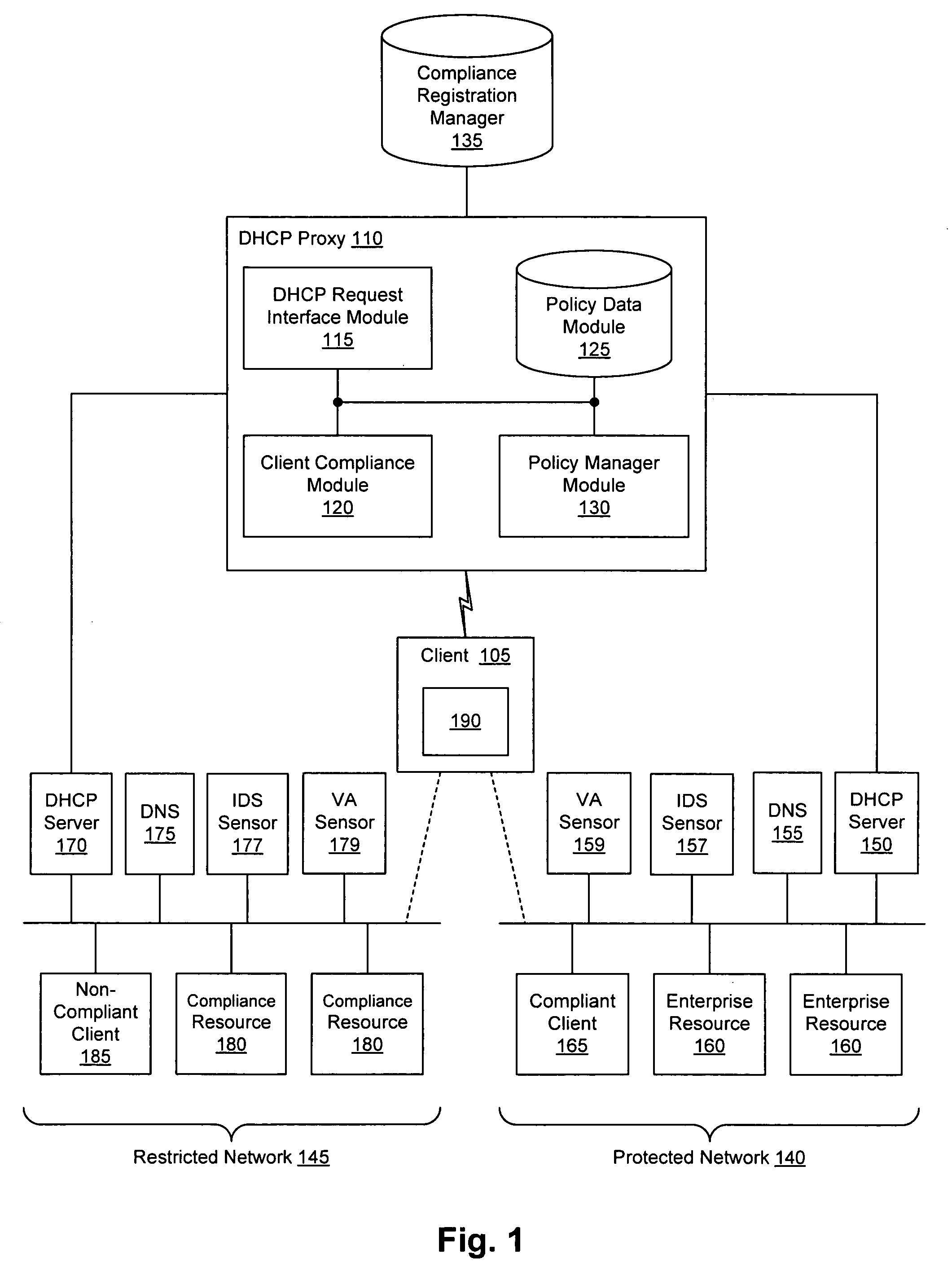
Client compliancy with self-policing clients
InactiveUS20060130139A1Memory loss protectionDigital data processing detailsNon complianceClient compliance
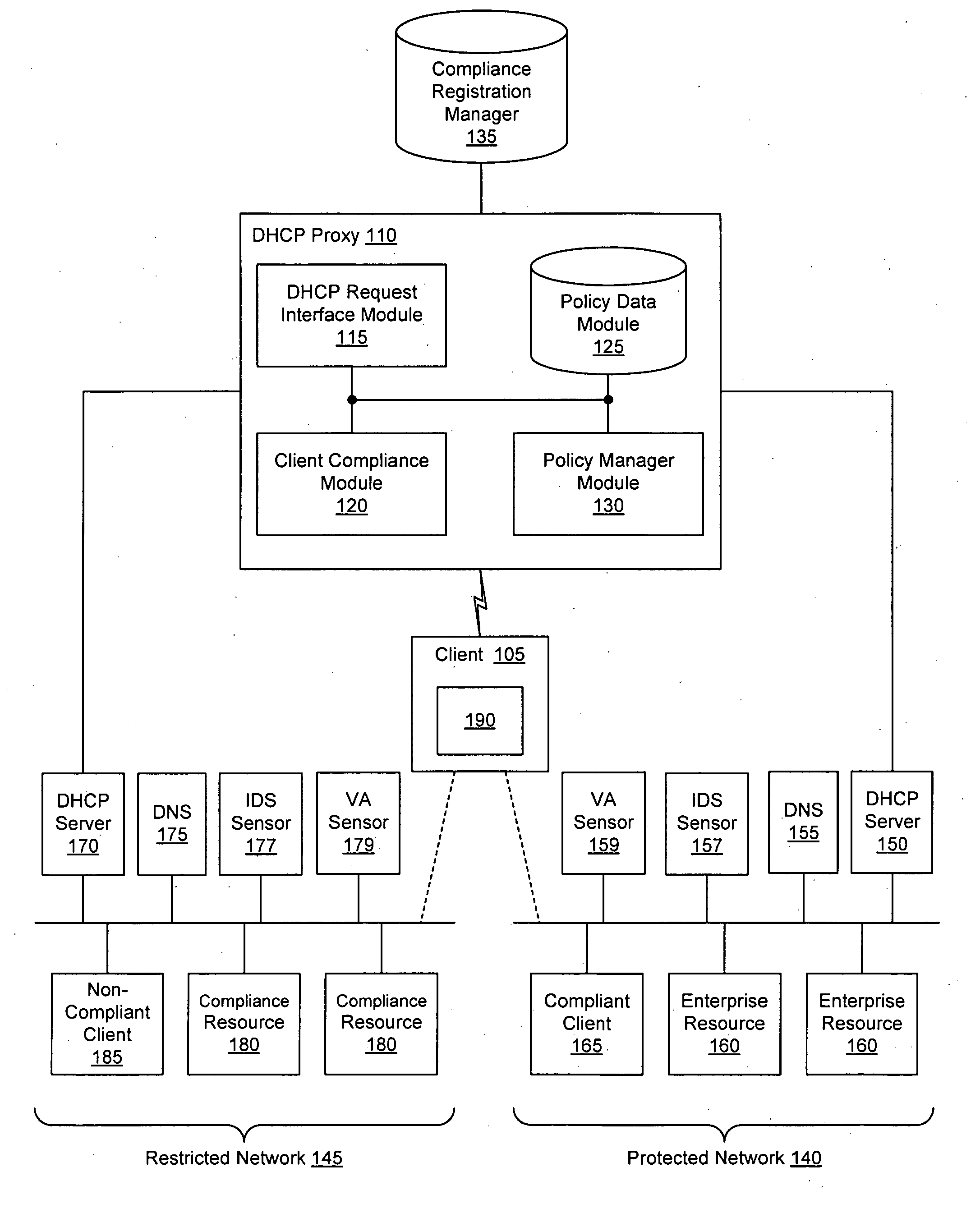
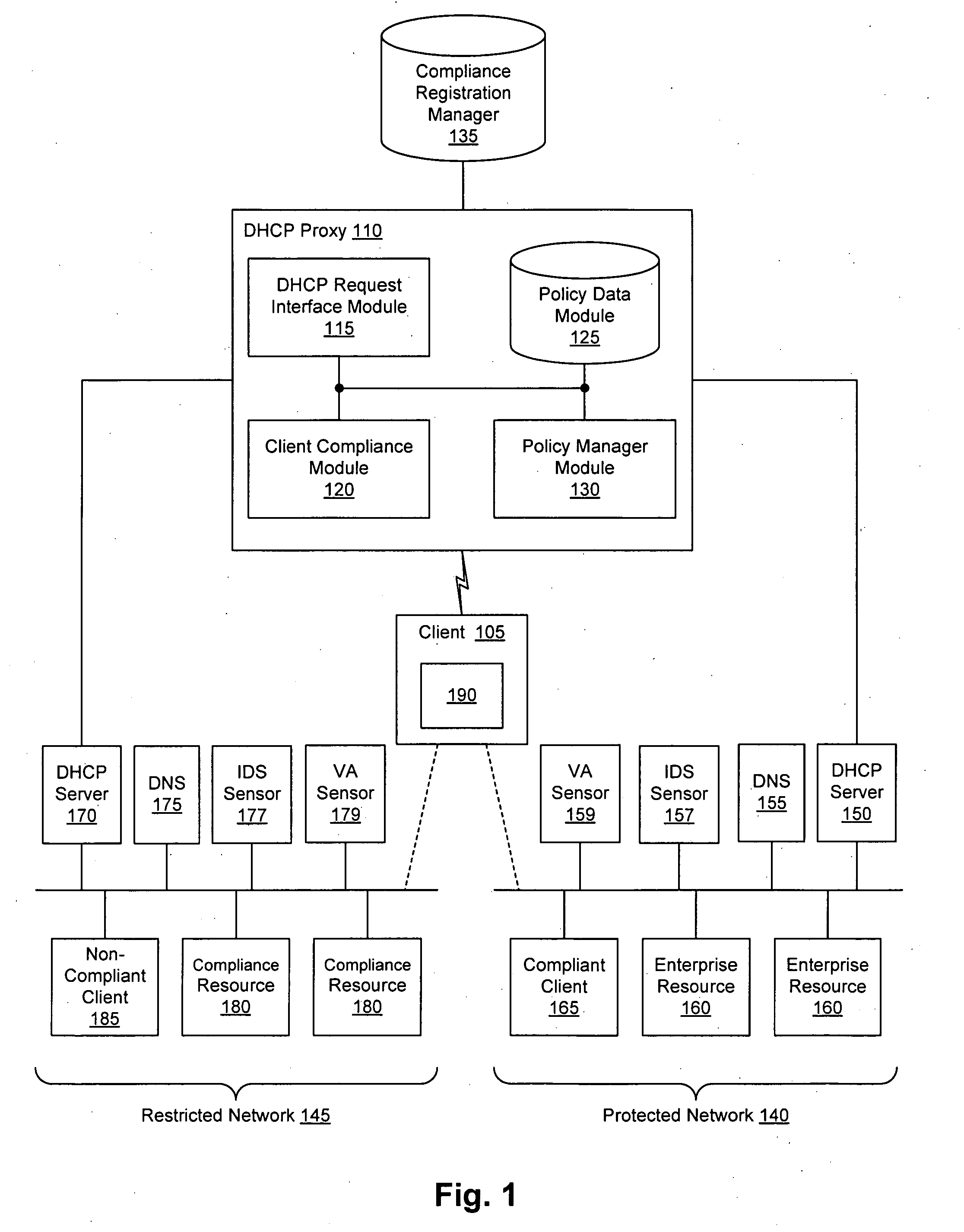
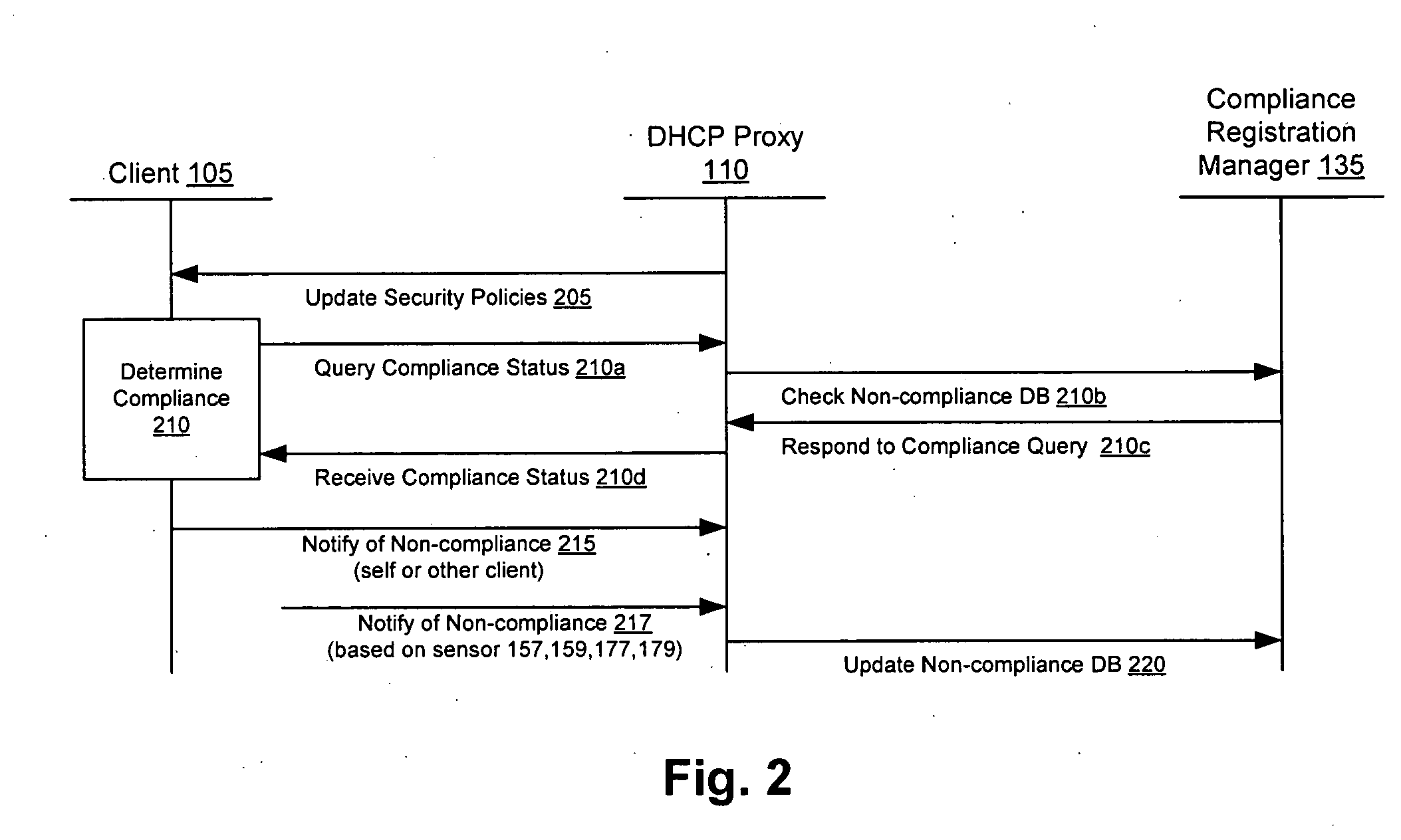
Security sensor data from intrusion detection system (IDS) sensors, vulnerability assessment (VA) sensors, and / or other security sensors is used to enhance the compliancy determination in a client compliancy system. A database is used to store the security sensor data. In one particular embodiment, a list of device compliance statuses indexed by corresponding identifiers (e.g., IP / MAC addresses) combined from IDS, VA, and / or other security sensing technologies is made available as a non-compliance database for query, so that clients and other compliancy authentication elements can tell that a particular client appears to be out of compliance. A client-side self-policing compliance system is enabled, and can be used in conjunction with automated endpoint compliance policy configuration to reduce system administrator burden.
Owner:CA TECH INC
System and method for managing global risk
InactiveUS20020129221A1Level of risk is minimizedLoss of competitive advantageGeneral purpose stored program computerOffice automationRisk statusNon compliance
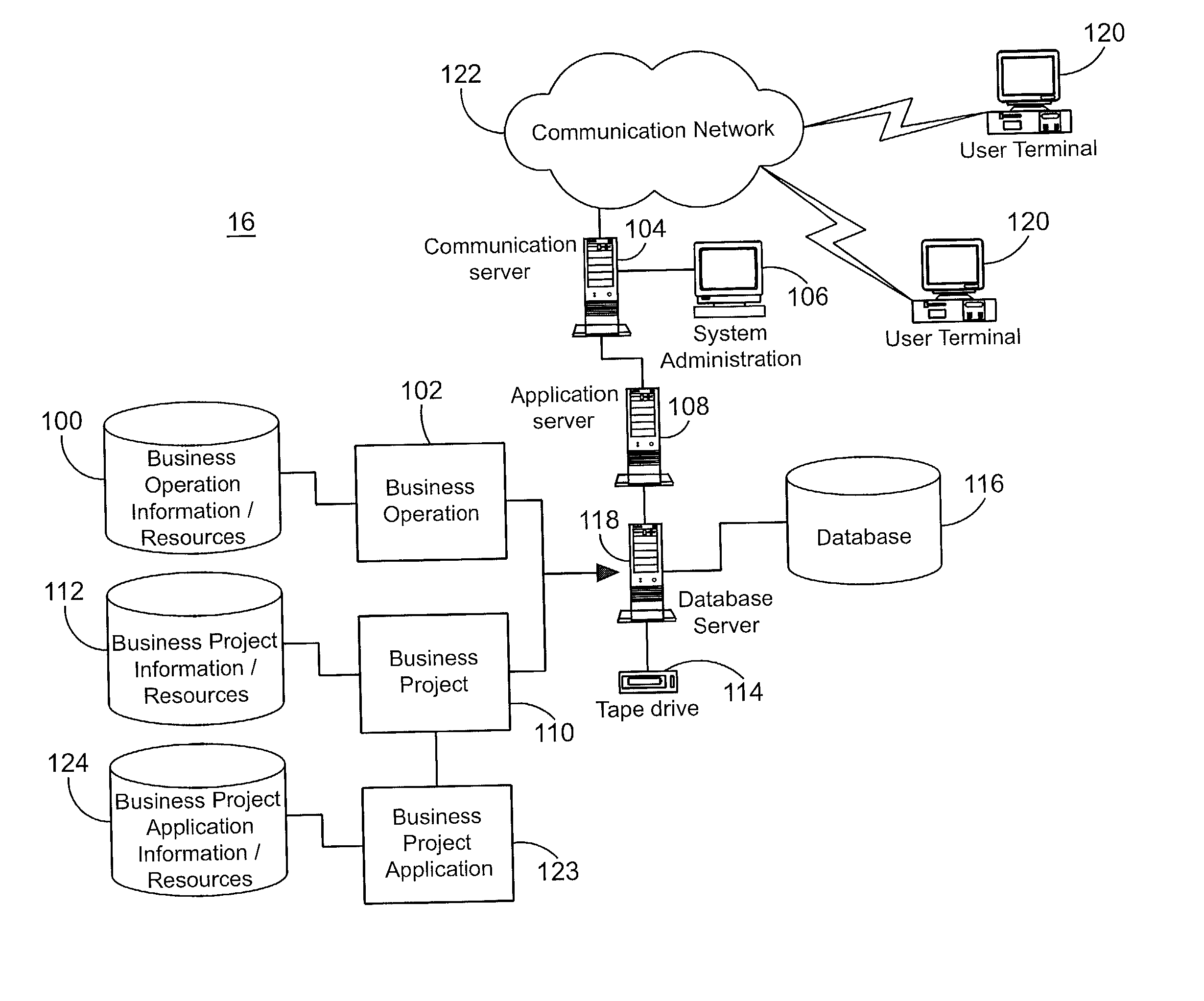
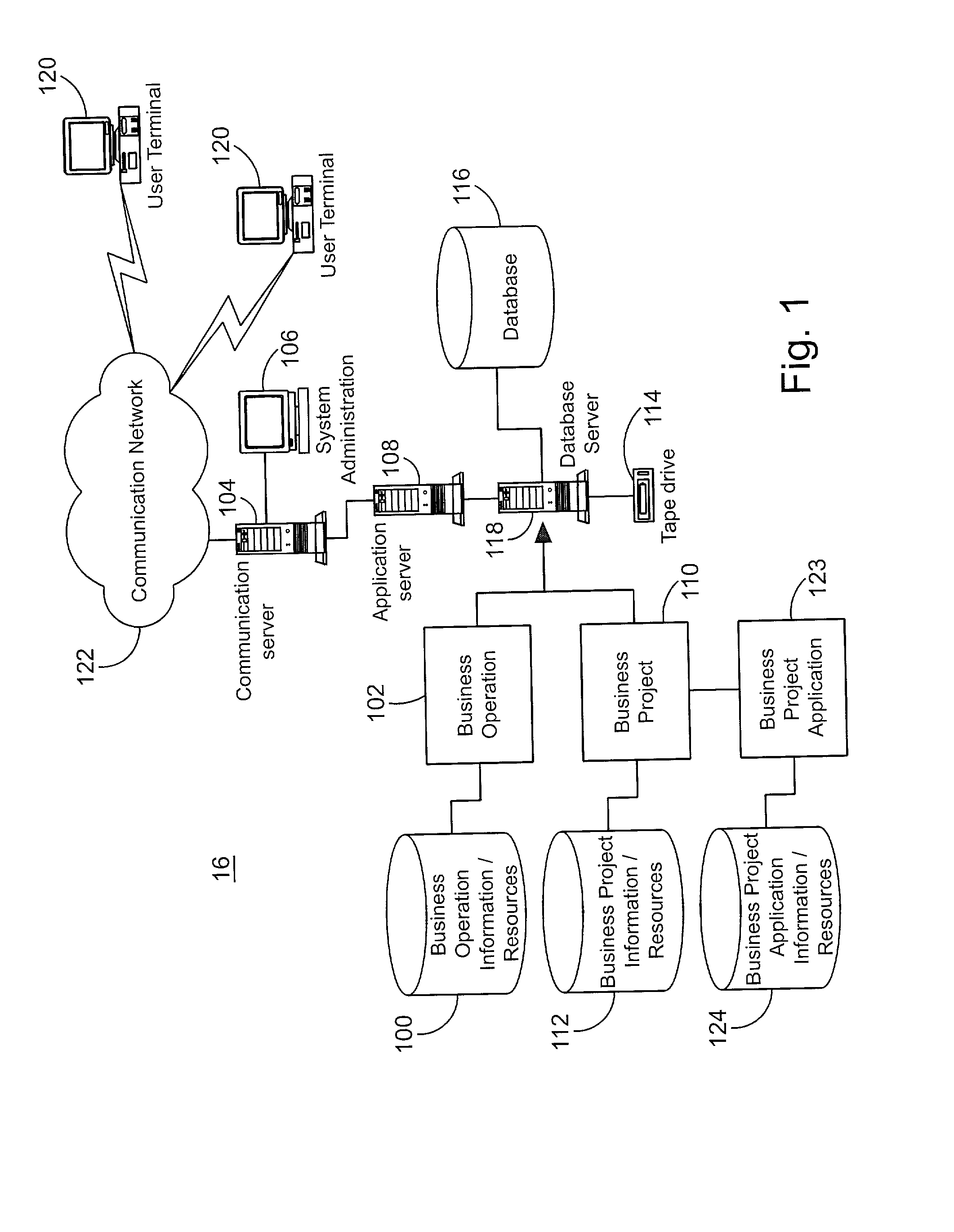
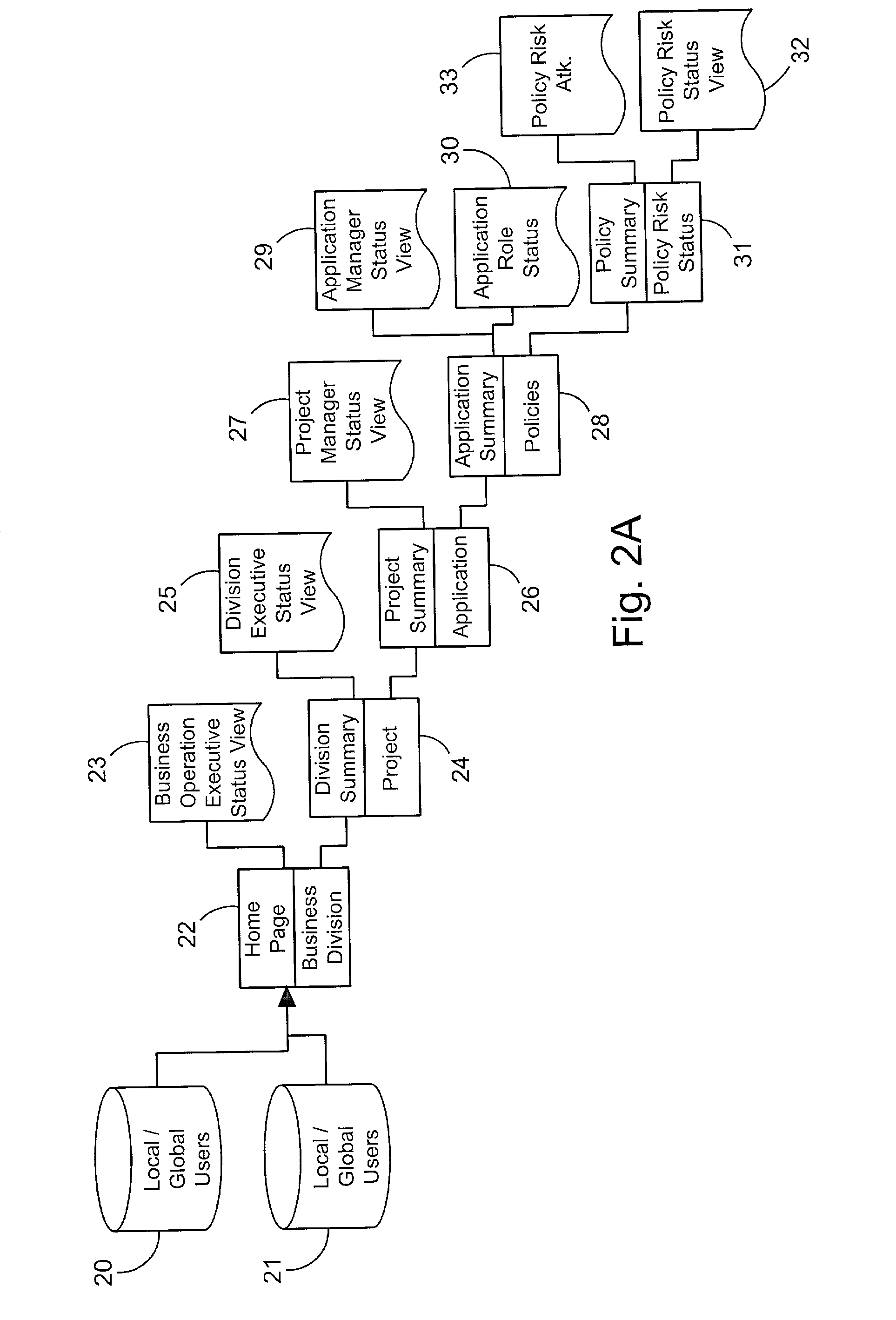
A system for tracking compliance with policies related to management of risk for a given enterprise provides risk status feedback on a number of managerial levels. The system notifies users of potential problems with non-compliance of enterprise policies set on a high level of enterprise management, and prompts the users to take steps to achieve compliance. The enterprise policies are designed to protect the enterprise from various forms of risk associated with enterprise activities. Accordingly, minimizing risk across enterprise operations, subdivisions, projects and applications produces an overall benefit of reduced liability or exposure to liability for the entire enterprise. A compliance status is provided by business groups at all levels of the enterprise, and consolidated for each management level to which the risk status is promoted. Higher level managers can view summaries of risk management status for the business divisions, and select particular statuses to view the condition of compliance among various business groups for which the manager has responsibility.
Owner:JPMORGAN CHASE BANK NA
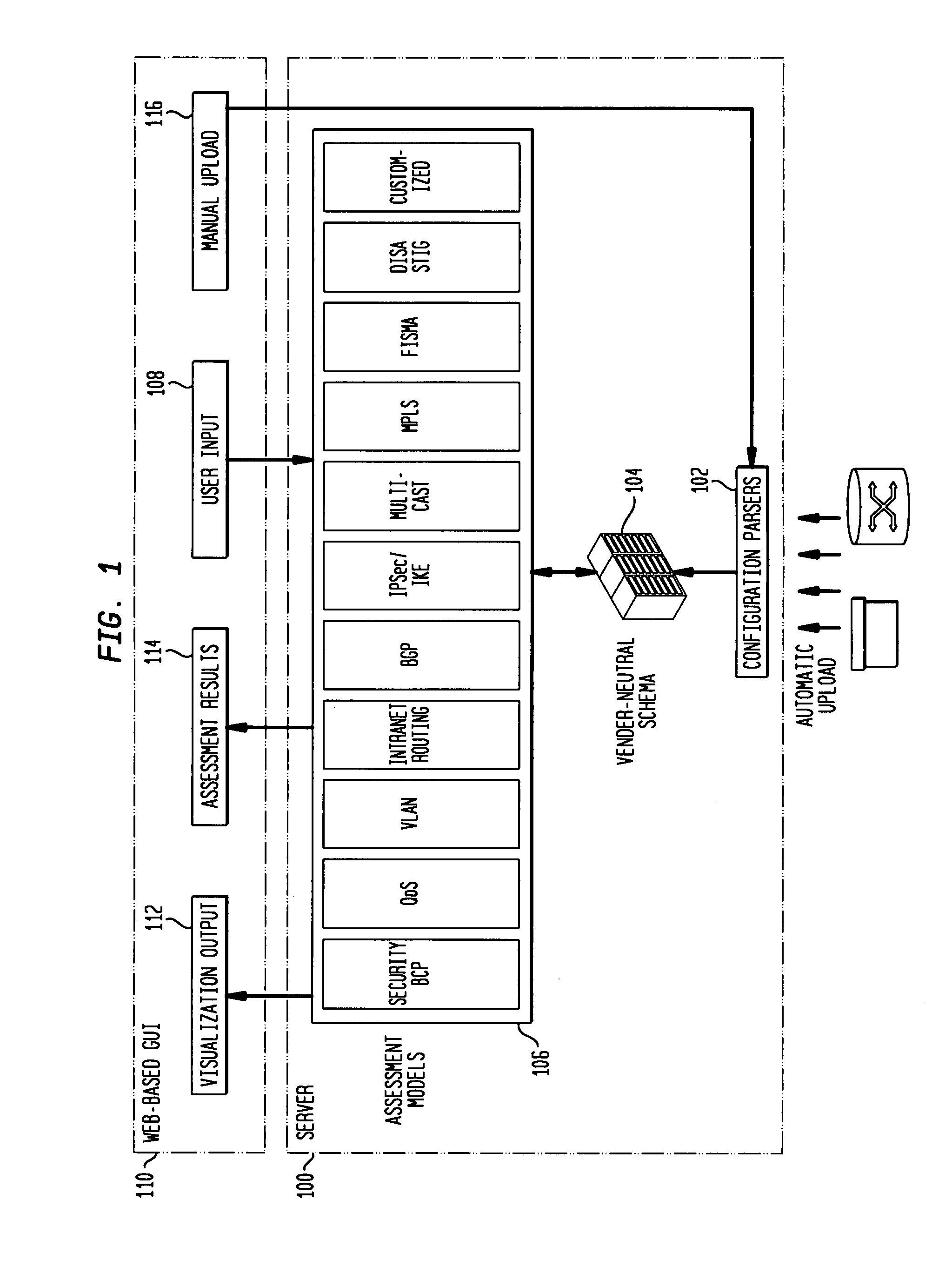
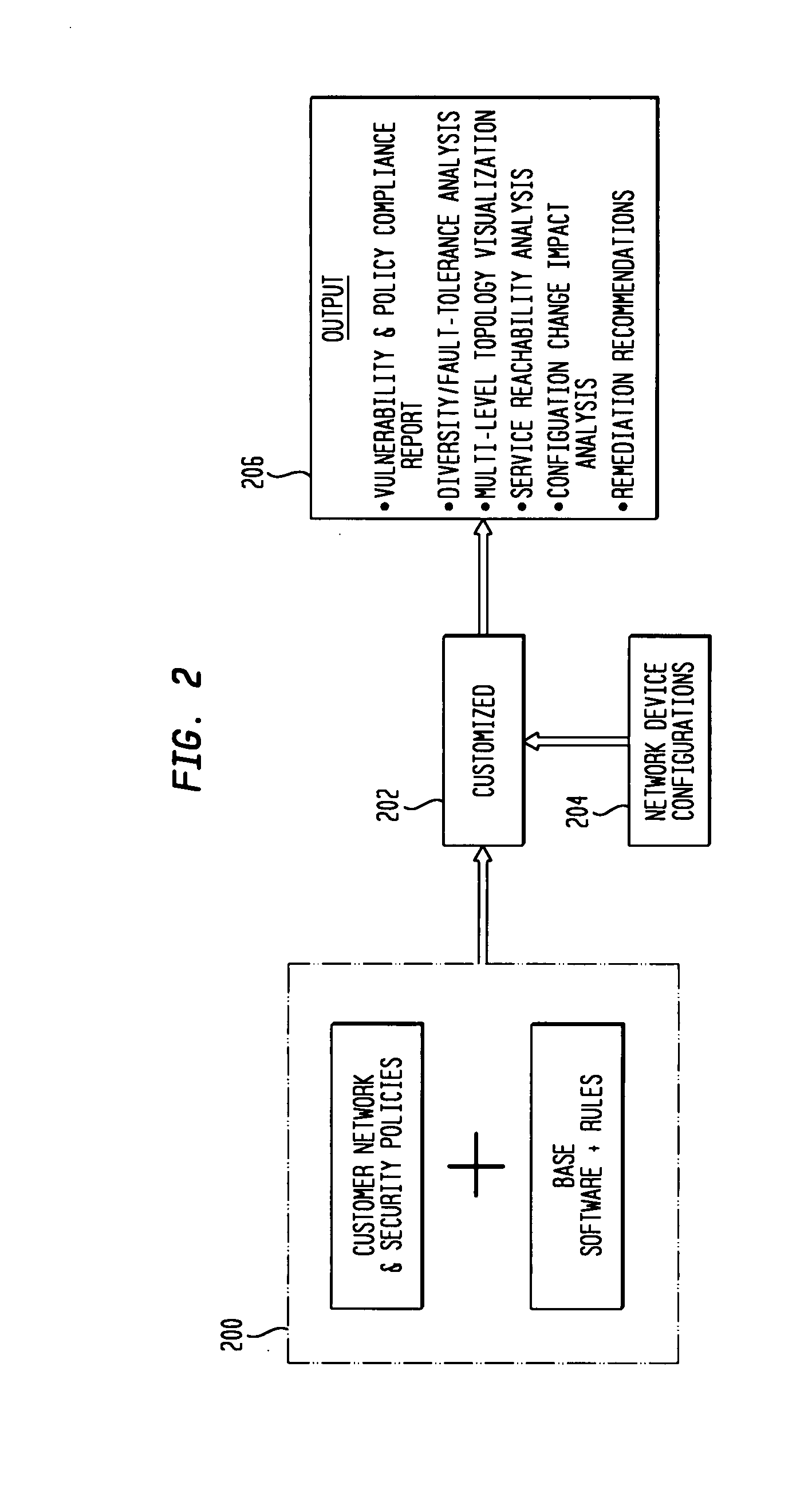
IP network vulnerability and policy compliance assessment by IP device analysis
InactiveUS20080172716A1Reduce vulnerabilityImprove network securityTransmissionSpecial data processing applicationsNetwork security policyCompliance problem
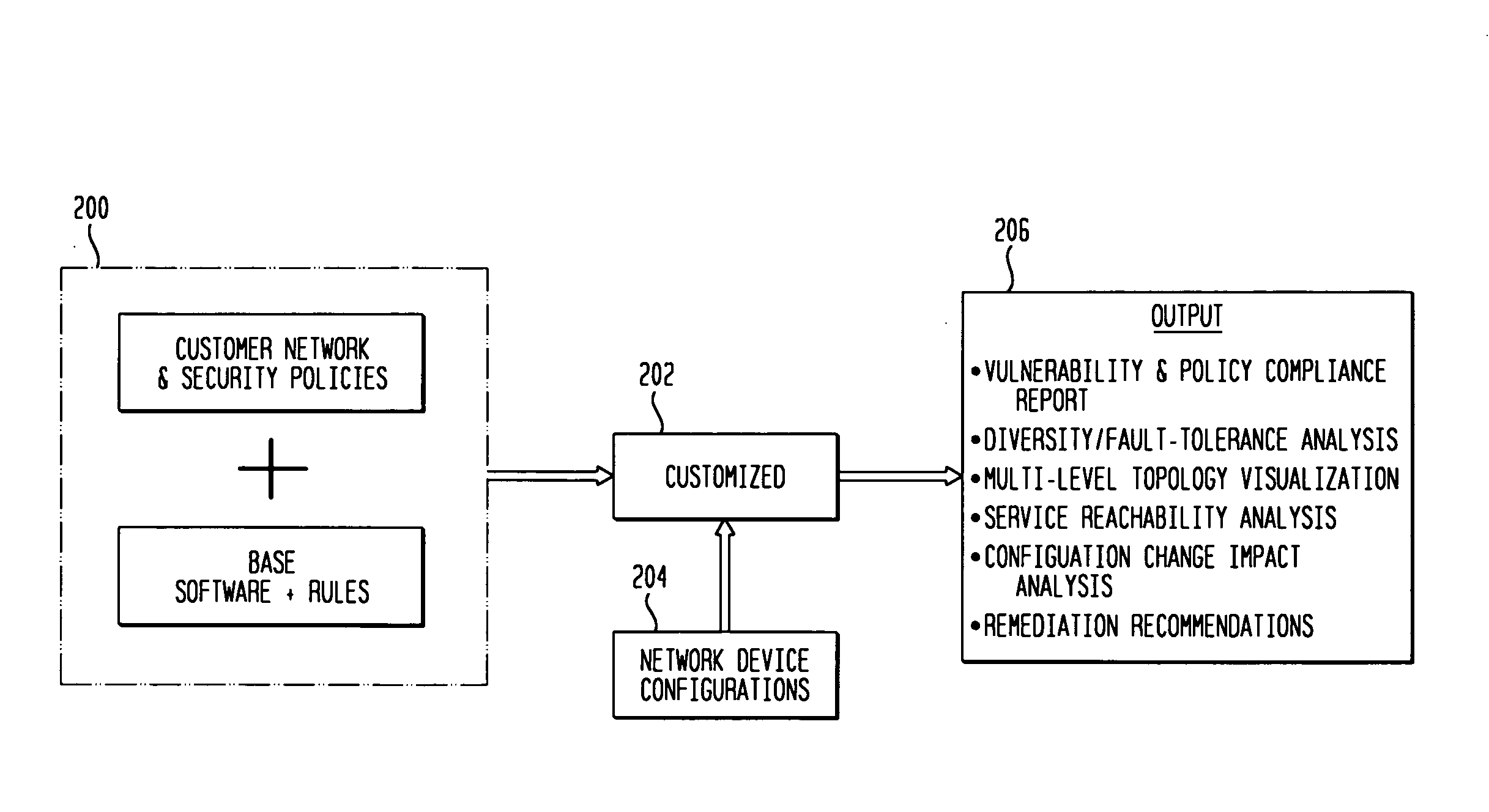
Customizable software provides assurances about the ability of an IP network to satisfy security, regulatory and availability requirements by comprehensive vulnerability and compliance assessment of IP networks through automated analysis of configurations of devices such as routers, switches, and firewalls. The solution comprises three main approaches for testing of IP device configurations to eliminate errors that result in vulnerabilities or requirements compliance issues. The first two fall in to the “static constraint validation” category since they do not change significantly for each IP network, while the last approach involves incorporation of each specific IP network's policies / requirements. These approaches are complementary, and may be used together to satisfy all the properties described above. The first approach involves checking the configurations of devices for conformance to Best-Current-Practices provided by vendors (e.g. Cisco Network Security Policy) and organizations such as the NIST, NSA or CERT. Also this includes checks of compliance with regulations such as FISMA, SOX, HIPPA, PCI, etc. The second approach is where as one reads device configurations, one collects beliefs about network administrator intent. As each belief is collected, an inference engine checks whether the new belief is inconsistent with previously accumulated beliefs. The third approach addresses the multiple device / protocol issue by including an understanding of high-level service and security requirements about the specific IP network under test from the network administrators.
Owner:TT GOVERNMENT SOLUTIONS
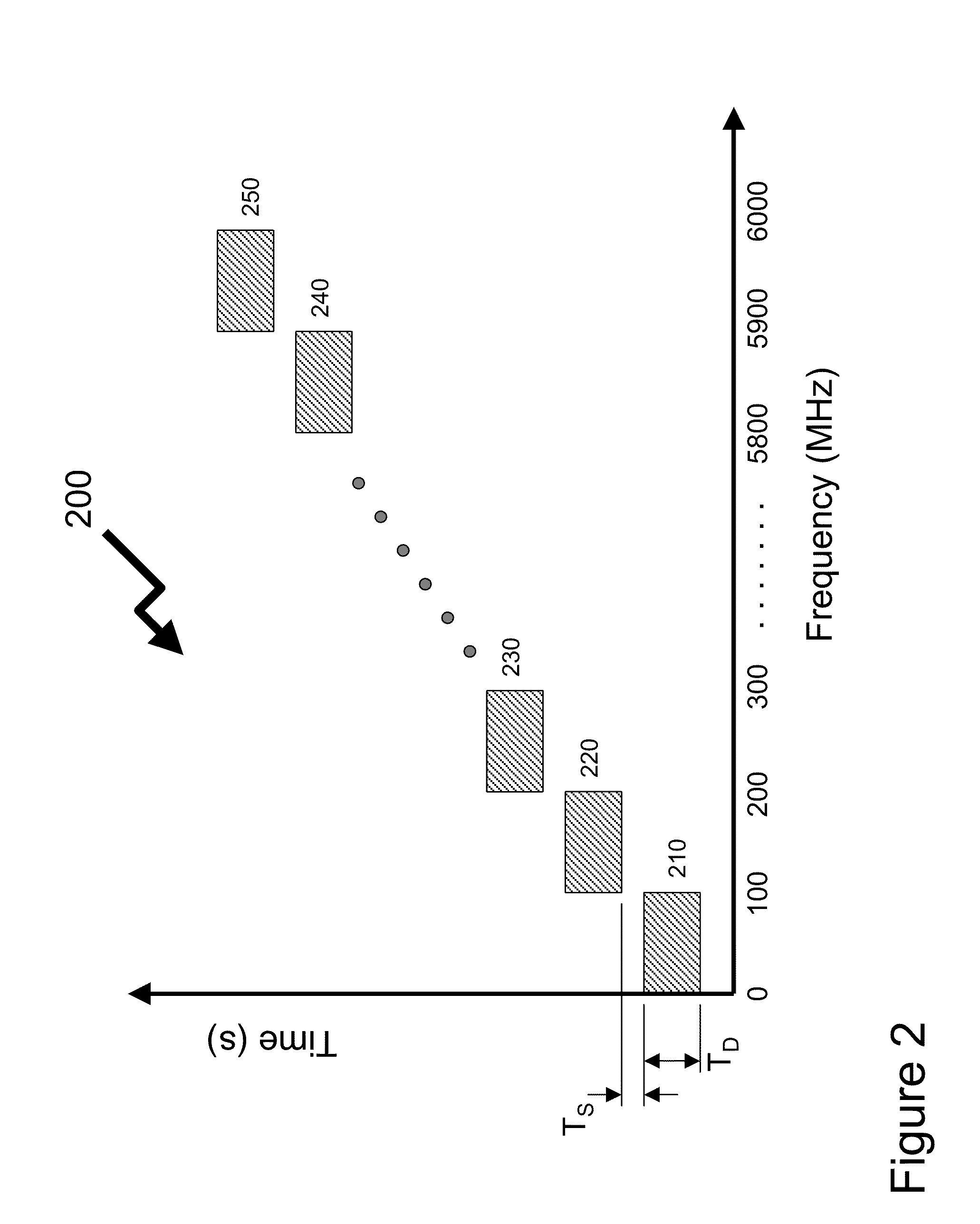
System and method for detecting RF transmissions in frequency bands of interest across a geographic area
ActiveUS20110185059A1Reduce communication overheadLow costNetwork topologiesDigital computer detailsFrequency spectrumNon compliance
Wireless devices form a significant portion of equipment forming the source / destination of content transmitted over telecommunications infrastructure together with applications such as RF identification, smart tags, etc. As such the wireless spectrum supports these devices operating to multiple standards, both licensed and unlicensed. In many environments it would be beneficial for a network administrator to know whether the environment and network they are responsible for is compliant to policies established in dependence of the environment / network. The invention provides distributed wireless signal analyzers within the environment / network to provide signal / spectrum analysis and determine whether received signals by the wireless signal analyzer are compliant to the network administrator policy. Compliance may be based upon time or frequency domain measurements with different rules for different wireless spectrum regions. Non-compliance is communicated to remote servers and / or network administrator and allows local control of network equipment.
Owner:THINKRF CORP
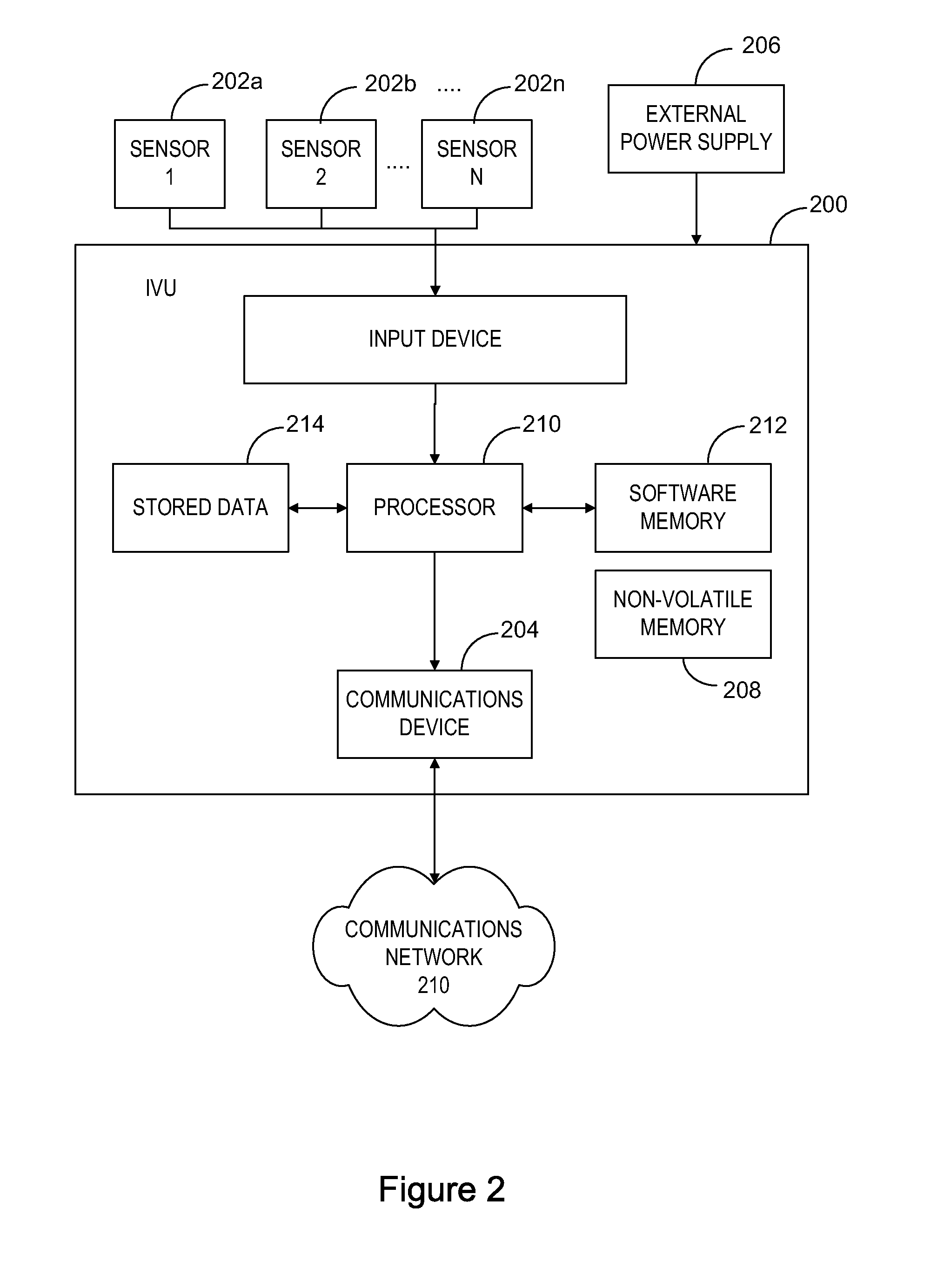
System for Monitoring Vehicle Use
ActiveUS20110035139A1Analogue computers for vehiclesAnalogue computers for trafficTime markNon compliance
A system for monitoring of vehicles and particularly heavy vehicles and their compliance with specific network (e.g. road) access conditions uses vehicle telematics solutions. The system includes an in-vehicle unit (IVU) associated with a vehicle being monitored. The IVU includes a receiver for receiving positioning signals, a processor for processing a time-marked log of vehicle data, a storage element for storing the time-marked log and a first wireless communication element for communicating time marked data to a Service Provider (SP) processing apparatus. One or more Service Providers operate Service Provider (SP) processing apparatus. The SP processing apparatus include a SP wireless communication element for receiving time-marked data from one or more IVUs and a SP processor for processing received data. The SP processor is adapted to compare received data from the time-marked log of a vehicle with one or more vehicle-use conditions applicable to that vehicle, and to generate a non-compliance report where the comparison indicates that non-compliant activity has occurred. A SP storage element stores non-compliance reports and relevant time-marked data.
Owner:TRANSPORT CERTIFICATION AUSTRALIA
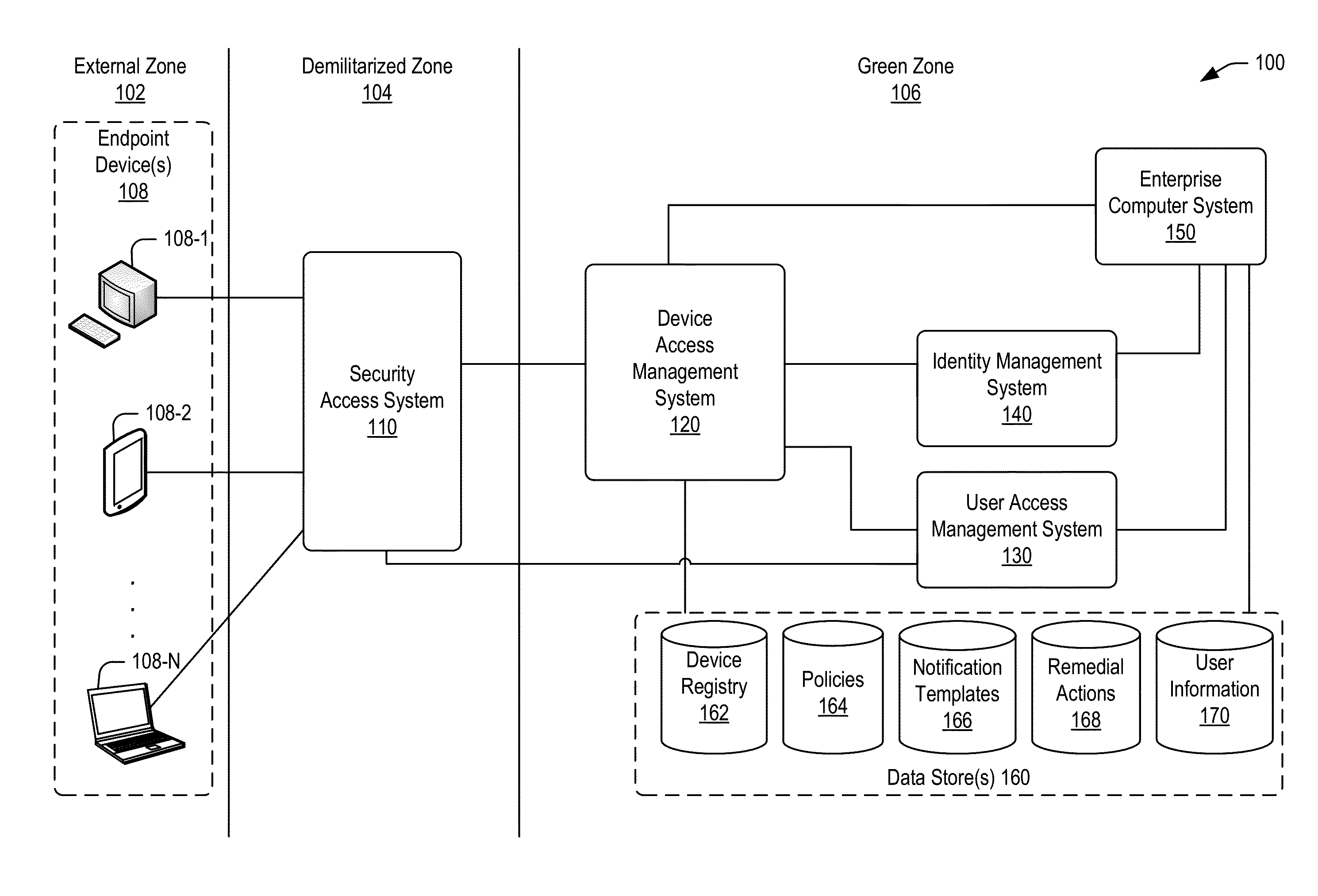
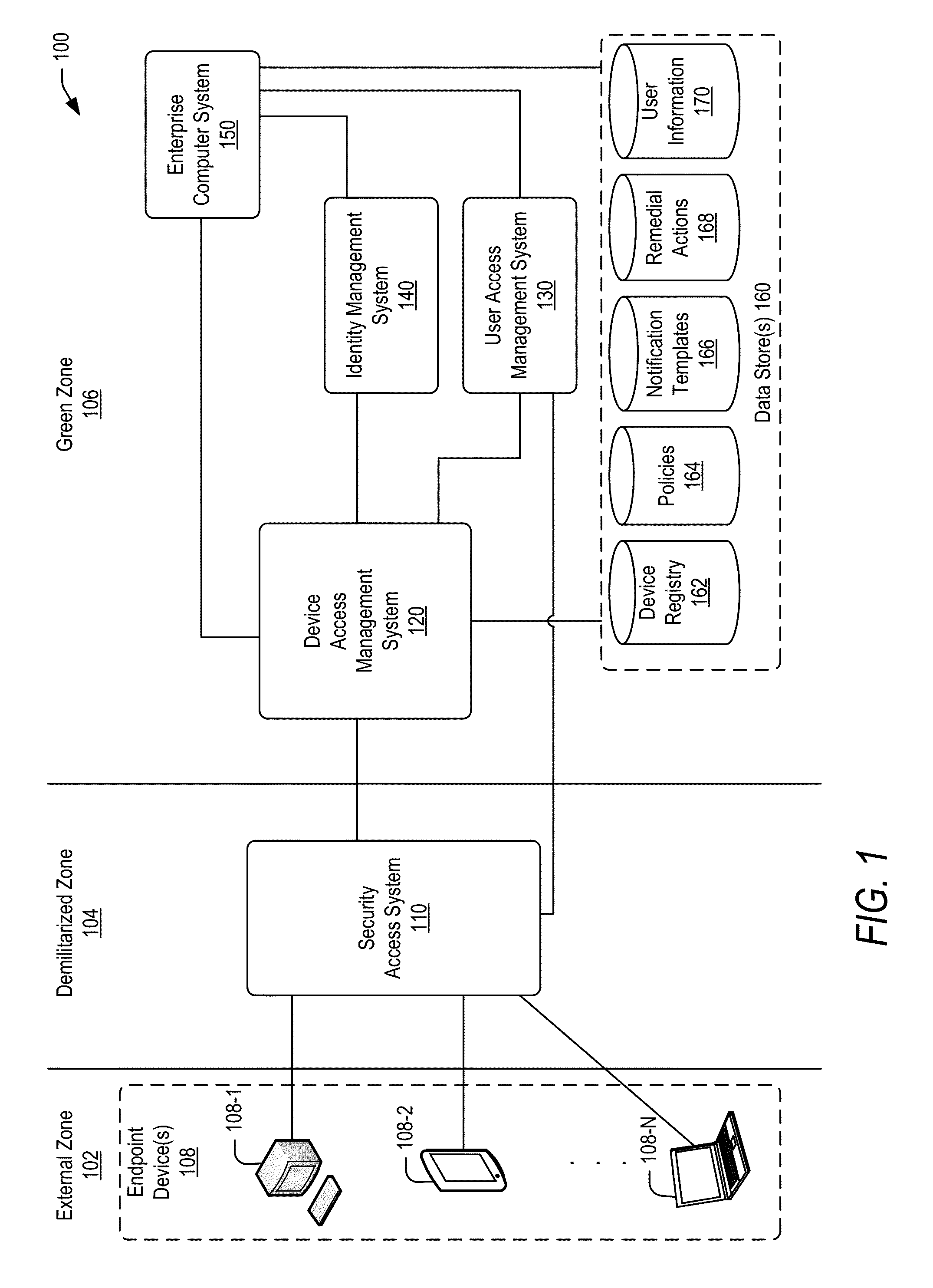
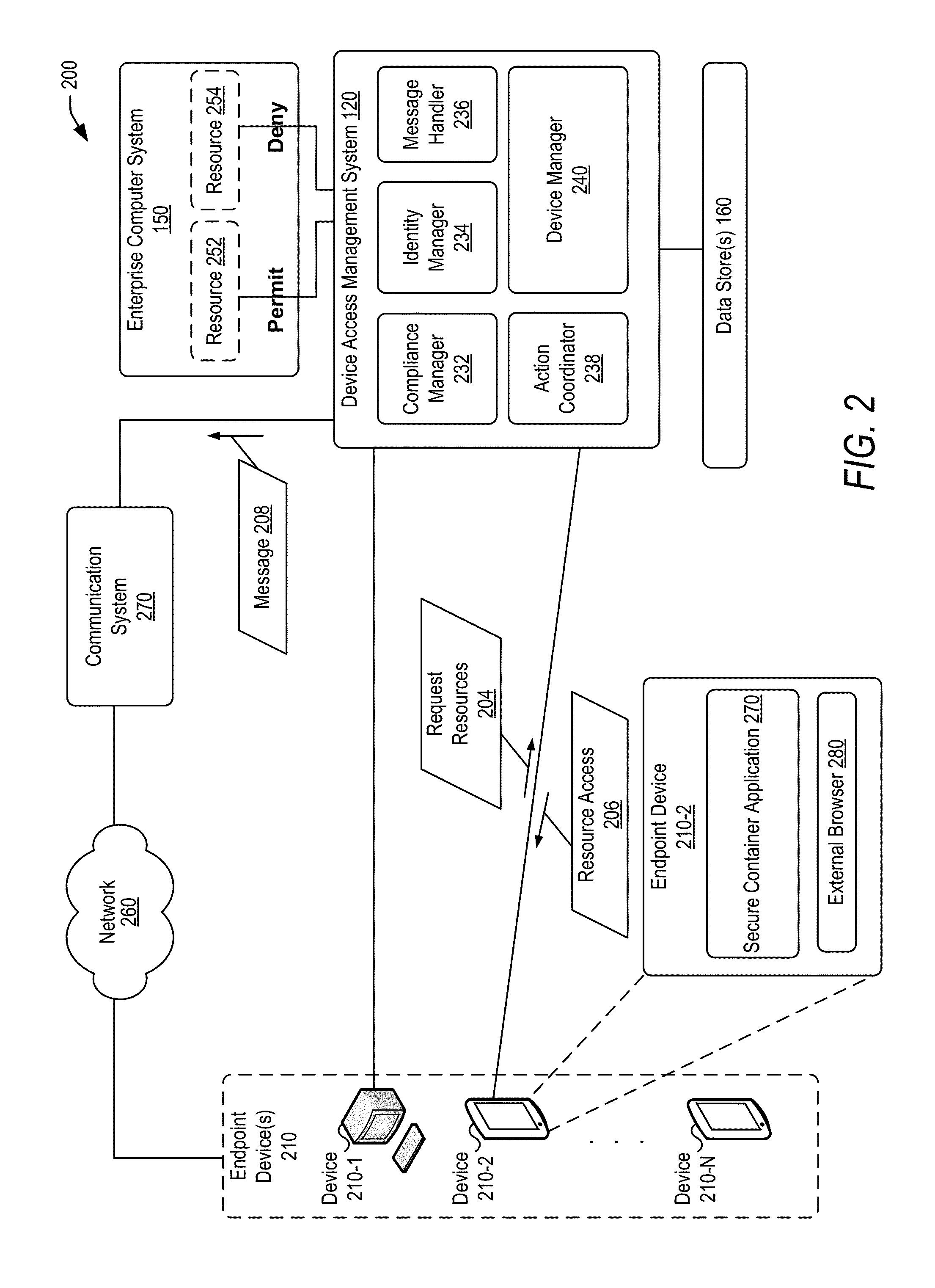
Policy-based compliance management and remediation of devices in an enterprise system
ActiveUS20160088021A1Reduce the burden onAvoid accessService provisioningVersion controlNon complianceEnterprise system
The present disclosure relates generally to managing compliance of remote devices that access an enterprise system. More particularly, techniques are disclosed for using a compliance policy to manage remediation of non-compliances of remote devices that access an enterprise system. A device access management system may be implemented to automate remediation of non-compliances of remote devices accessing an enterprise system. Remediation may be controlled based on different levels of non-compliance, each defined by one or more different non-compliances. In some embodiments, a level of non-compliance may be conditionally defined by one or more user roles for which non-compliance is assessed. Access to computing resources of an enterprise system may be controlled for a remote device based on compliance of the remote device. Access may be inhibited for those resources not permitted during a time period of a non-compliance.
Owner:ORACLE INT CORP
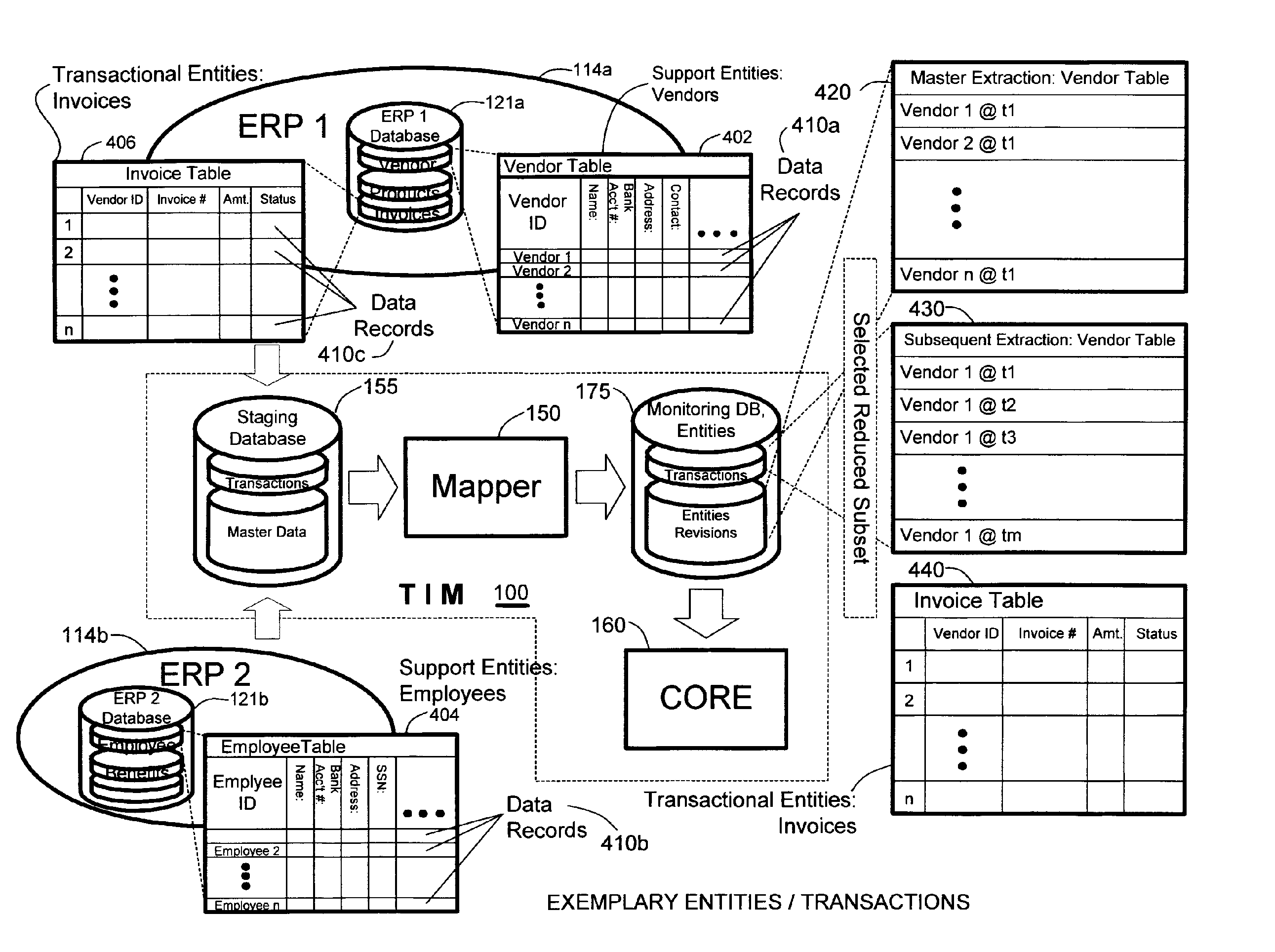
Methods and systems for monitoring transaction entity versions for policy compliance
ActiveUS20060212487A1Low costQuality improvementDigital data information retrievalFinanceData ingestionData source
A system for determining lack of compliance of a transactional entity with an enterprise policy by maintaining an historical record of the entity as changes are made over time. The system allows establishment, codification, and maintenance of enterprise policies, monitors electronic transactions of the enterprise from various data sources, detects exceptions to established policies, reports exceptions to authorized users such as managers and auditors, and / or provides a case management system for tracking exceptions and their underlying transactions. A master data extractor establishes an initial instance of a transactional entity in a monitoring database. A changed data extractor is responsive to changed data for establishing a subsequent instance of the transactional entity in the monitoring database. A transaction analysis engine applies predetermined policy rules to data in the monitoring database to determine lack of compliance of the initial and subsequent instances of the transactional entity with enterprise policies.
Owner:OVERSIGHT SYST INC
Apparatus and method for prediction and management of participant compliance in clinical research
InactiveUS20060184493A1Improve statistics performanceReduce testing costsDigital computer detailsForecastingCardiovascular drugNon compliance
A system for developing and implementing empirically derived algorithms to generate decision rules to determine participant noncompliance and fraud with research protocols in clinical trials allows for the identification of complex patterns of variables that detect or predict participant noncompliance and fraud with research protocol, including performance and enrollment goals, in the clinical trial. The data may be used to overall predict the performance of any participant in a clinical trial, allowing selection of participants that tend to produce useful, high-quality results. The present invention can also be used to monitor participant compliance with the research protocol and goals to determine preferred actions to be performed. Optionally, the invention may provide a spectrum of noncompliance, from minor noncompliance needing only corrective feedback, to significant noncompliance requiring participant removal from the clinical trial or from future clinical trials. The algorithms and decision rules can also be domain-specific, such as detecting non-compliance or fraud among subjects in a cardiovascular drug trial, or demographically specific, such as taking into account gender, age or location, which provides for algorithms and decision rules to be optimized for the specific sample of participants being studied.
Owner:ERESTECH
Compliance monitoring for anomaly detection
InactiveUS6983266B1Improve accuracyImprove efficiencyFuzzy logic based systemsKnowledge representationCompliance MonitoringTruth value
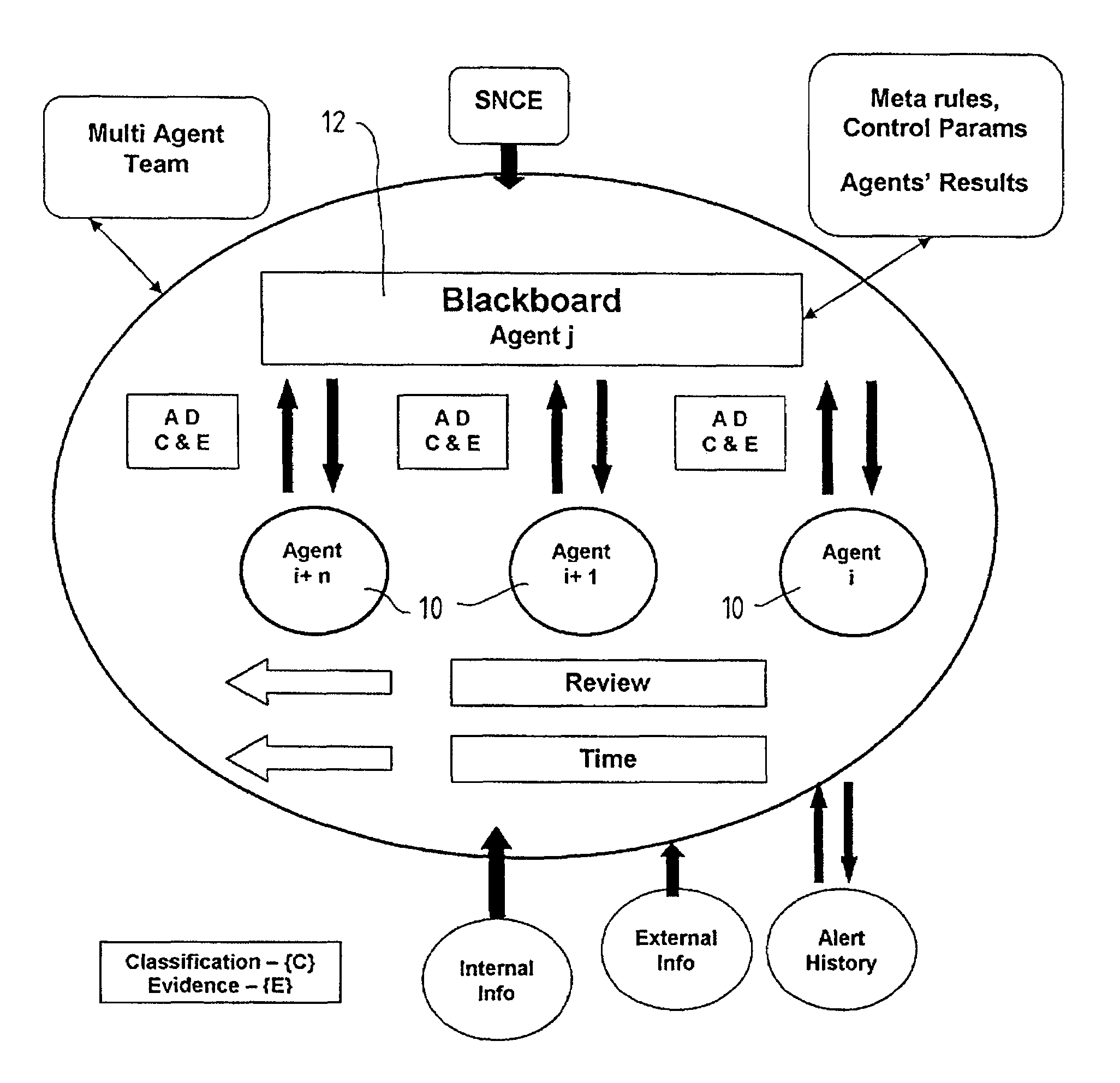
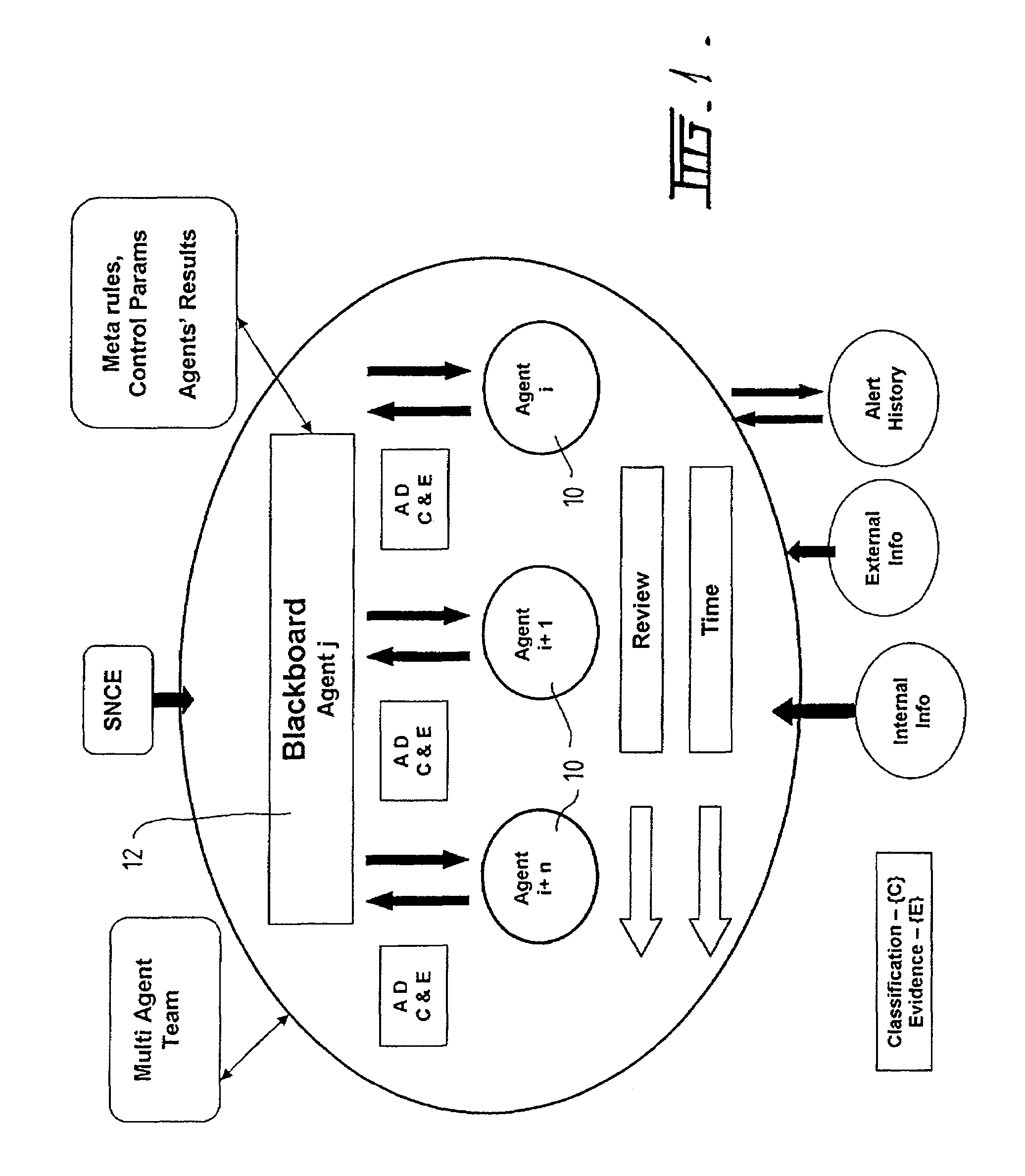
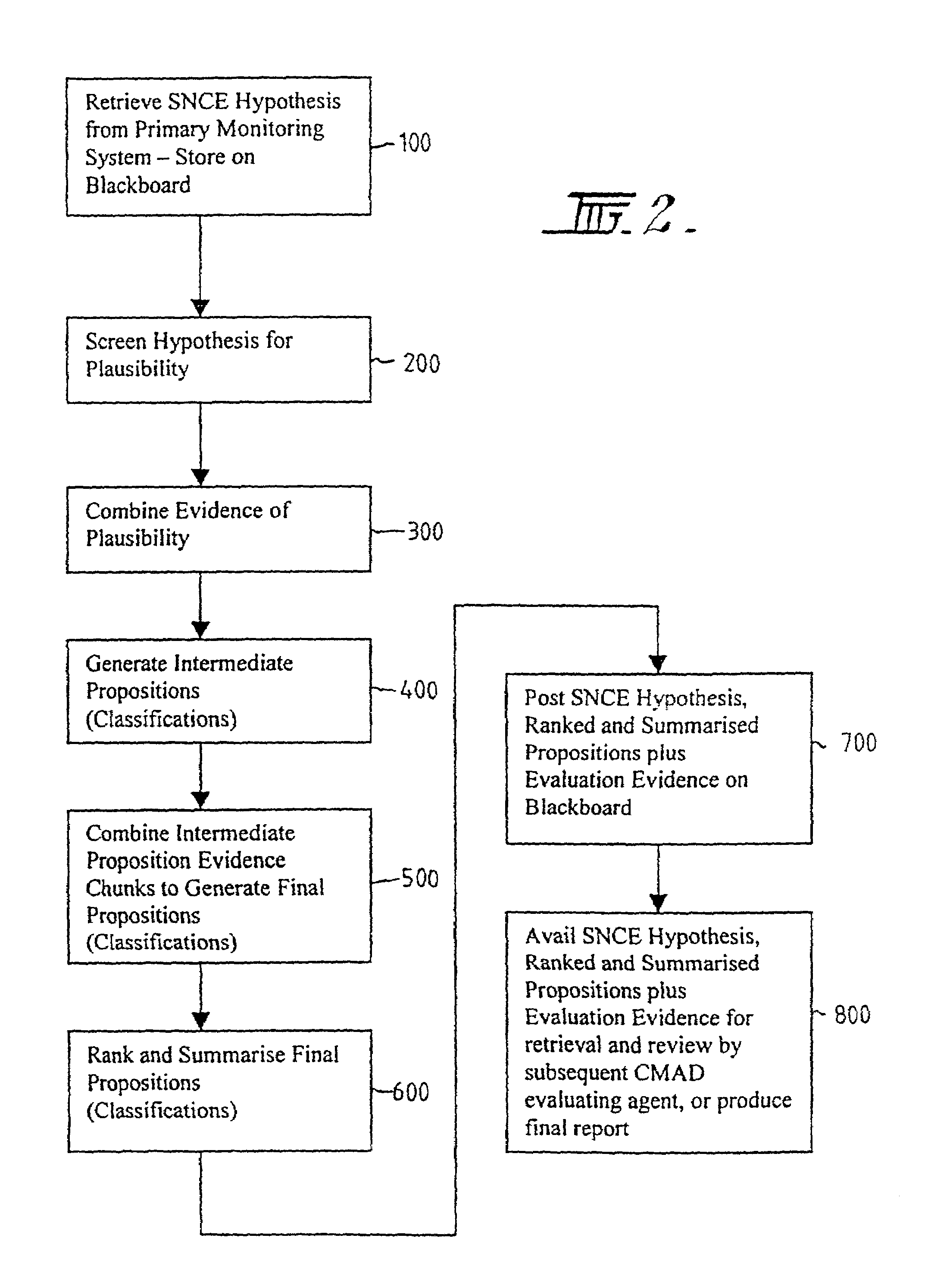
A method and system for supporting a compliance agent in compliance monitoring for anomaly detection (CMAD) involves a primary monitoring system comparing some predetermined conditions of acceptance with the actual data or event. If any variance is detected (an anomaly) by the primary monitoring system, an exception report or alert is produced, identifying the variance. In a simple environment, this identification of the variance fulfils the evidence conditions and determines an instance of non-compliance. However, in a more complex environment, it may only be an indicator of a suspect non-compliant event (SNCE). In the latter case, the compliance agent uses the results of the initial monitoring as well as important information related to the event and requiring judgmental expertise to obtain further evidence of non-compliance. Compliance gents develop propositions or believes, based on their assumption. For each proposition node in the system, the assumption based truth maintenance system maintains a list of minimum sets of assumptions (Boolean cues), which are relevant to the SNCE type. At the macro level, the construct uses the trivalent belief-disbelief-unknown. However, this is refined by applying a measure of importance to individual pieces or empirical evidence.
Owner:ALERT KM
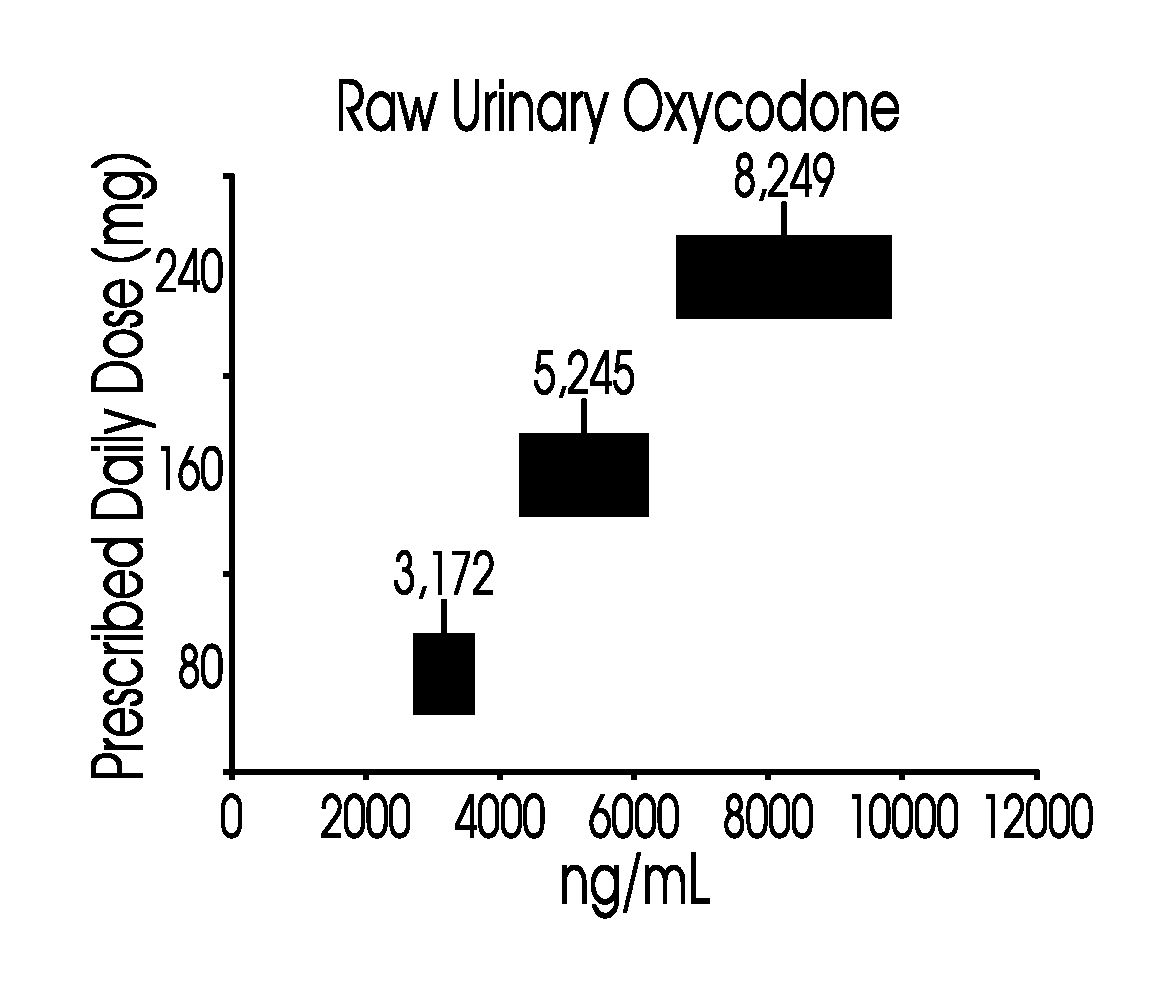
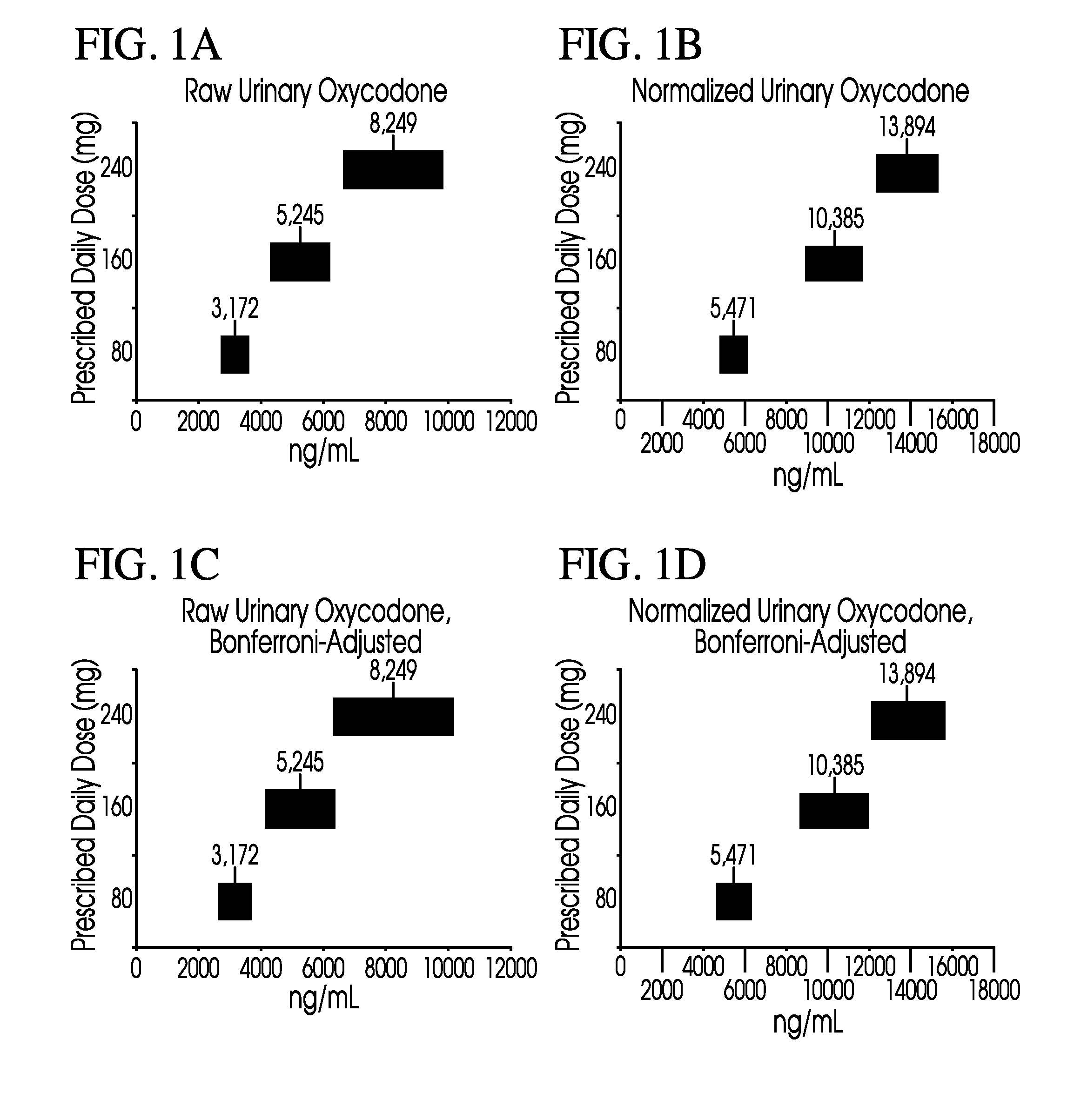
Methods of normalizing measured drug concentrations and testing for non-compliance with a drug treatment regimen
Methods for monitoring subject compliance with a prescribed treatment regimen are disclosed. In one embodiment, the method comprises measuring a drug level in fluid of a subject and normalizing said measured drug level as a function of one or more parameters associated with the subject. The normalized drug level is compared to a reference value and associated confidence intervals or to a concentration range. The reference value and associated confidence intervals and / or the concentration range may be normalized based on one or more parameters associated with subjects in a reference population.
Owner:AMERITOX LLC
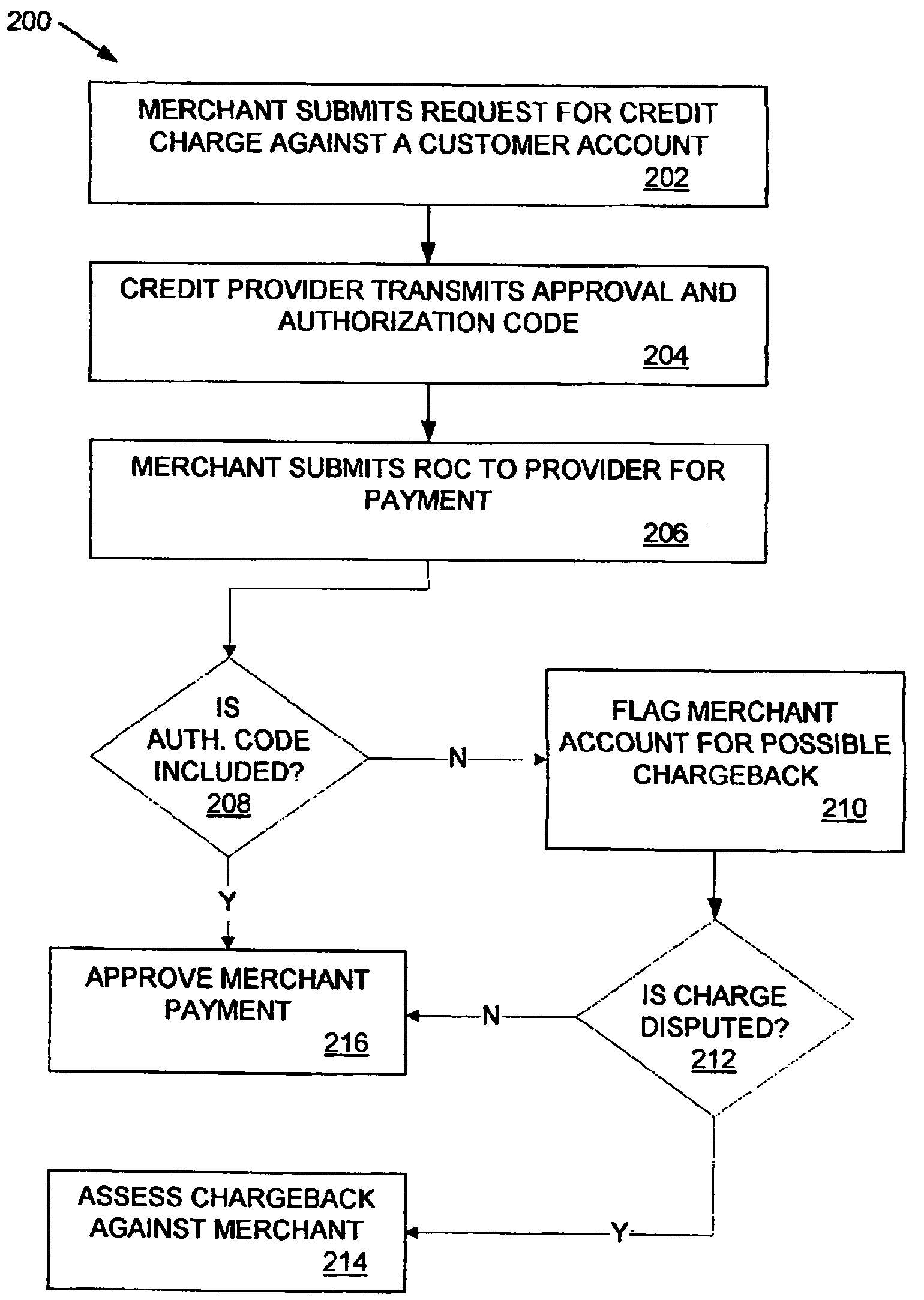
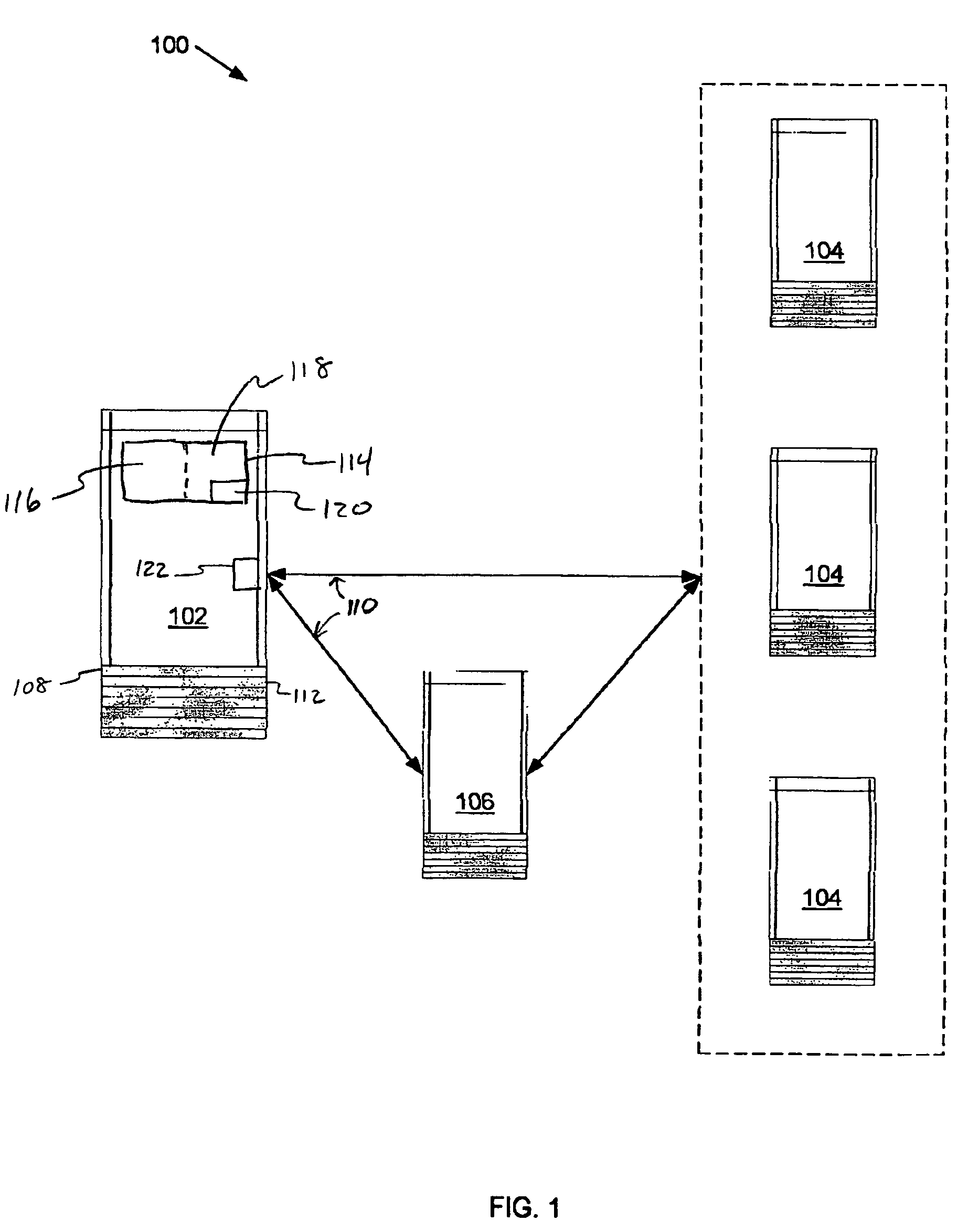
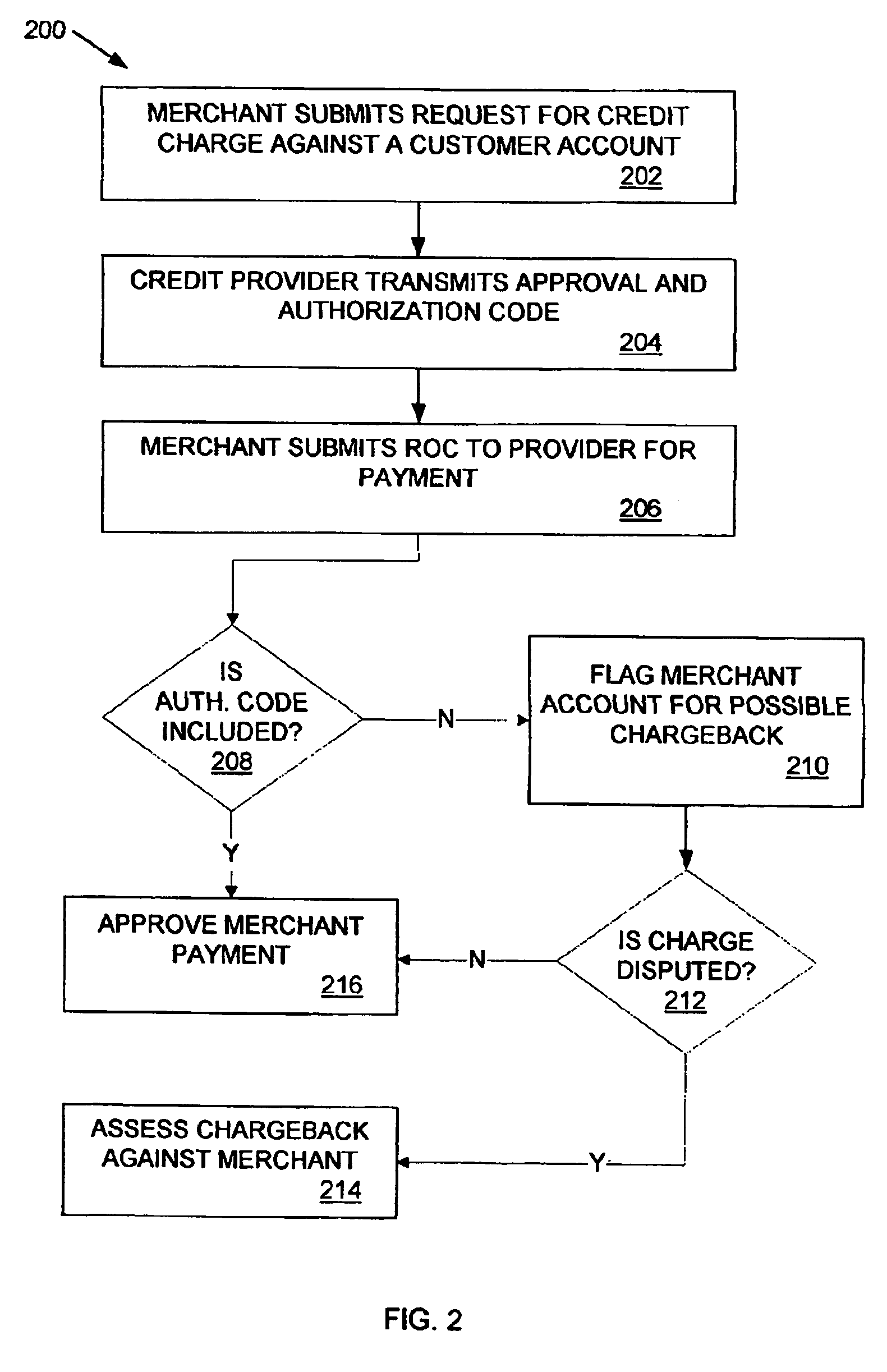
Method and apparatus for reducing fraudulent credit transactions by requiring merchant return of multi-digit authorization codes
Processes for reducing fraudulent credit transactions, including financial (e.g., credit, charge, debit, etc.) card transactions, are introduced, in which merchants receive multi-digit authorization codes from a credit provider (e.g., a customer transaction account card user) with all approved transactions. To guarantee payment, all merchants are required to later resubmit the multi-digit authorization code with every record of charge, regardless of dollar amount, as verification that an authorization was obtained. Merchants that fail to provide any authorization code, or an incorrect authorization code, will be subject to a chargeback for non-compliance.
Owner:LIBERTY PEAK VENTURES LLC
Enhanced client compliancy using database of security sensor data
InactiveUS20060070129A1Eliminate non-compliancyMemory loss protectionDigital data processing detailsNon complianceClient compliance
Security sensor data from intrusion detection system (IDS) sensors, vulnerability assessment (VA) sensors, and / or other security sensors is used to enhance the compliancy determination in a client compliancy system. A database is used to store the security sensor data. In one particular embodiment, a list of device compliance statuses indexed by corresponding identifiers (e.g., IP / MAC addresses) combined from IDS, VA, and / or other security sensing technologies is made available as a non-compliance database for query, so that clients and other compliancy authentication elements can tell that a particular client appears to be out of compliance. A client-side self-policing compliance system is enabled, and can be used in conjunction with automated endpoint compliance policy configuration to reduce system administrator burden.
Owner:CA TECH INC
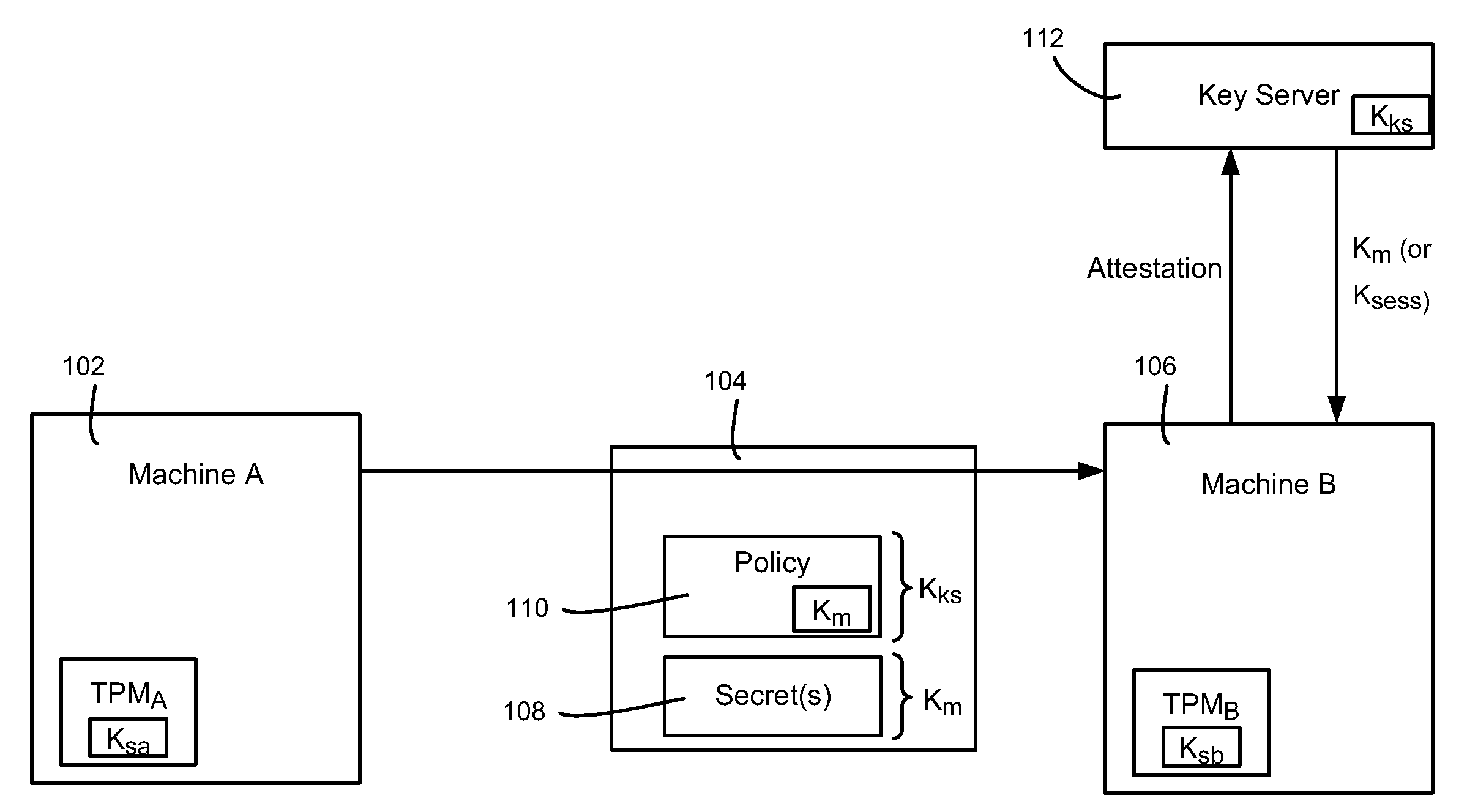
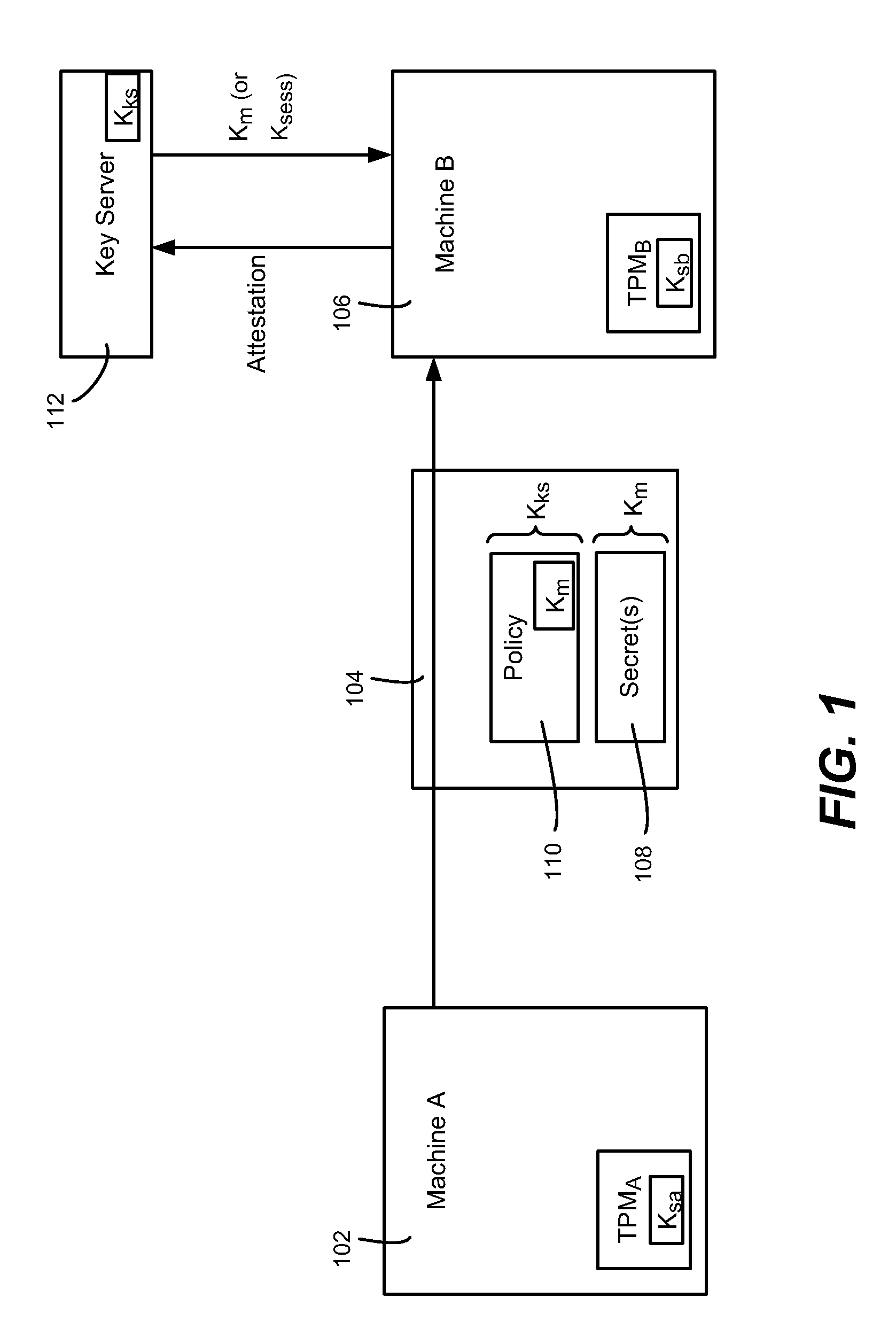
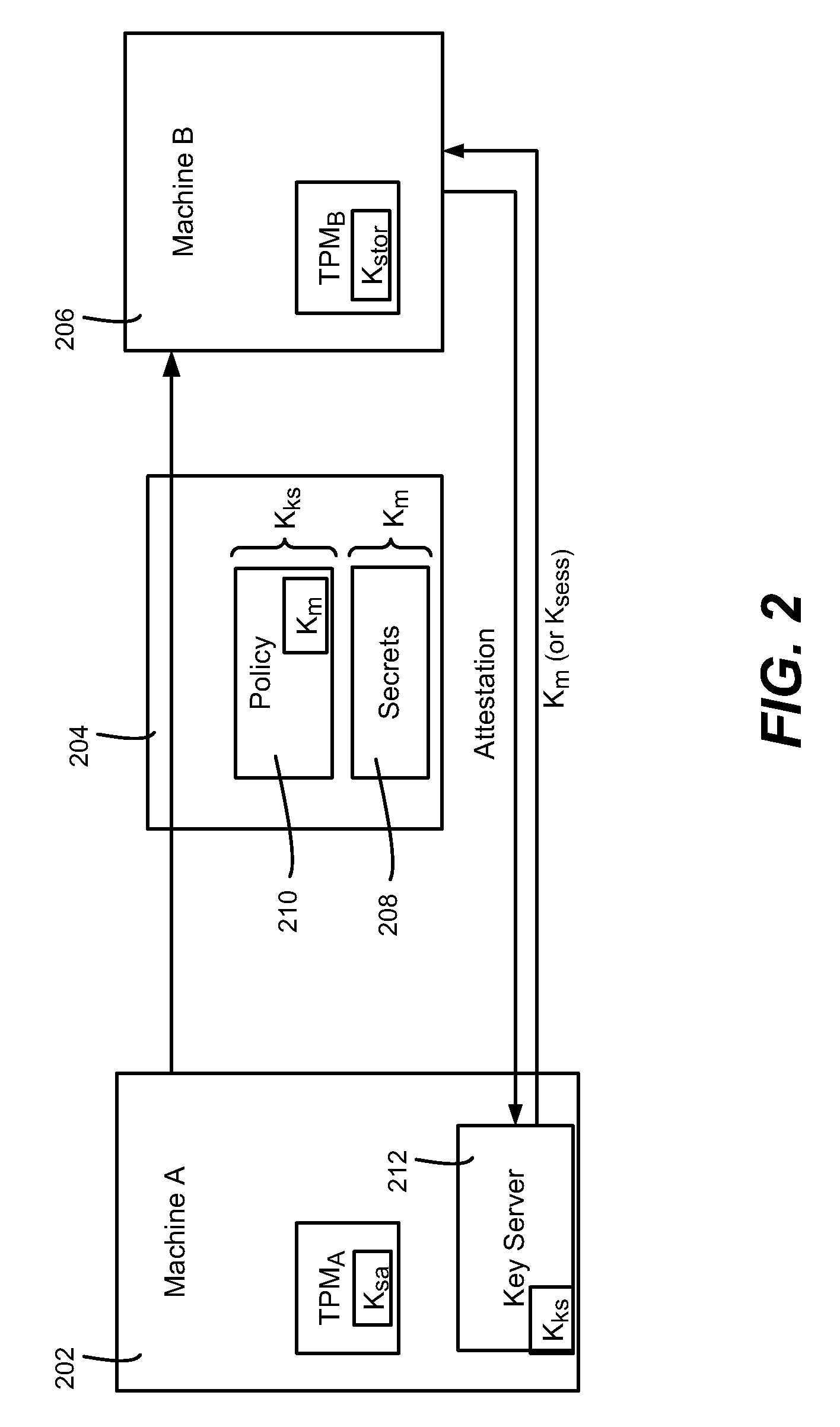
Migration of computer secrets
ActiveUS20090154709A1Well formedKey distribution for secure communicationUser identity/authority verificationComputer hardwareTrusted Platform Module
Described is a technology by which computer data secrets sealed by a trusted platform module (TPM) or like device may be securely migrated from a physical source computing machine to a physically different destination machine. For example, migration of TPM secrets allows migration of a virtual machine from one physical machine to another. A destination machine receives a set of data sealed at a source machine. The set of data includes a migration key and a secret sealed by the migration key. The destination machine performs attestation with a key server to attest that the destination machine is entitled to access the sealed secret, via credentials, known good configuration and / or other policy compliance. The key server unseals the migration key, and provides a returned key (e.g., the migration key or a session key) to the destination machine for unsealing the secrets.
Owner:MICROSOFT TECH LICENSING LLC
Measurement-based management method for packet communication networks
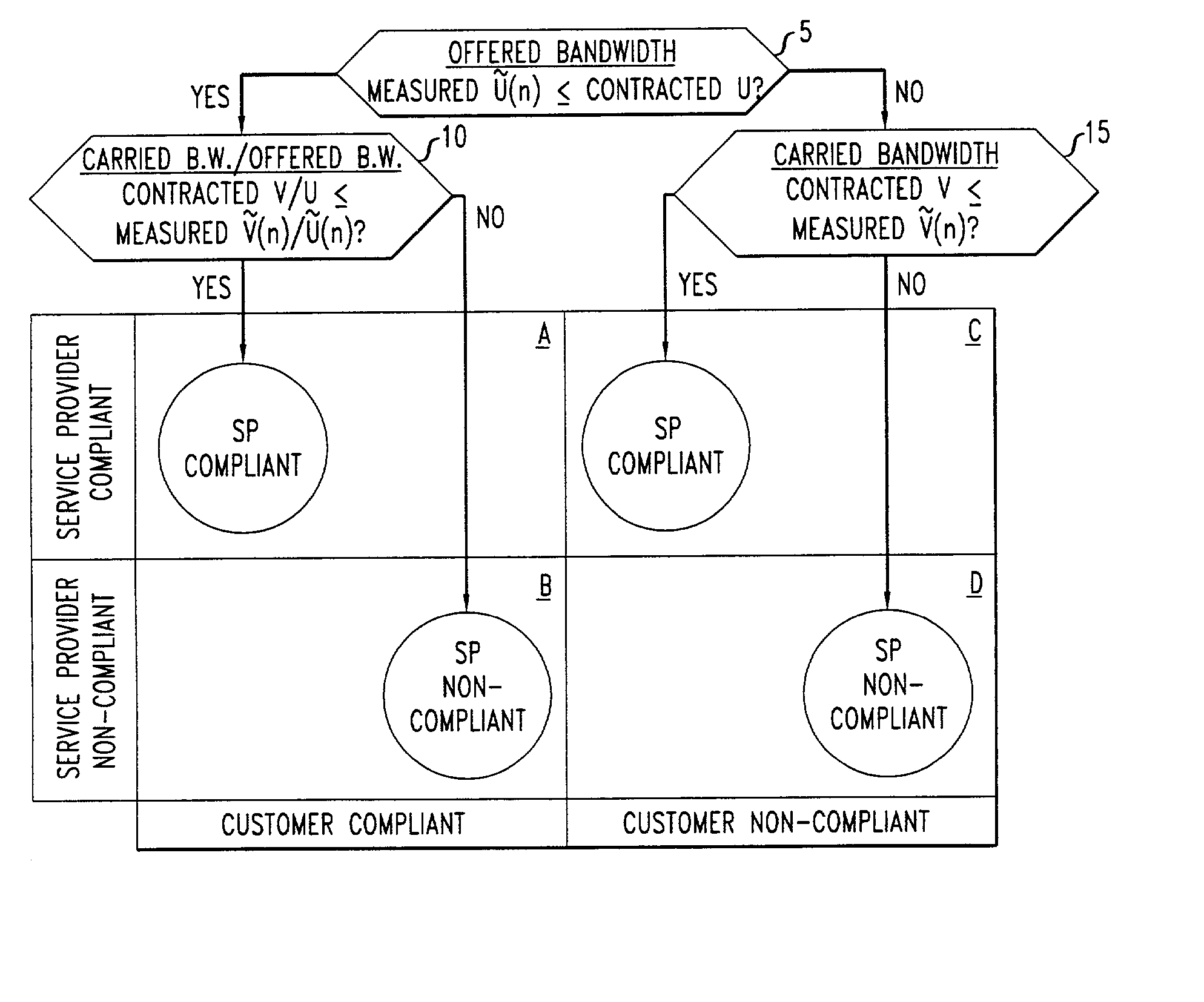
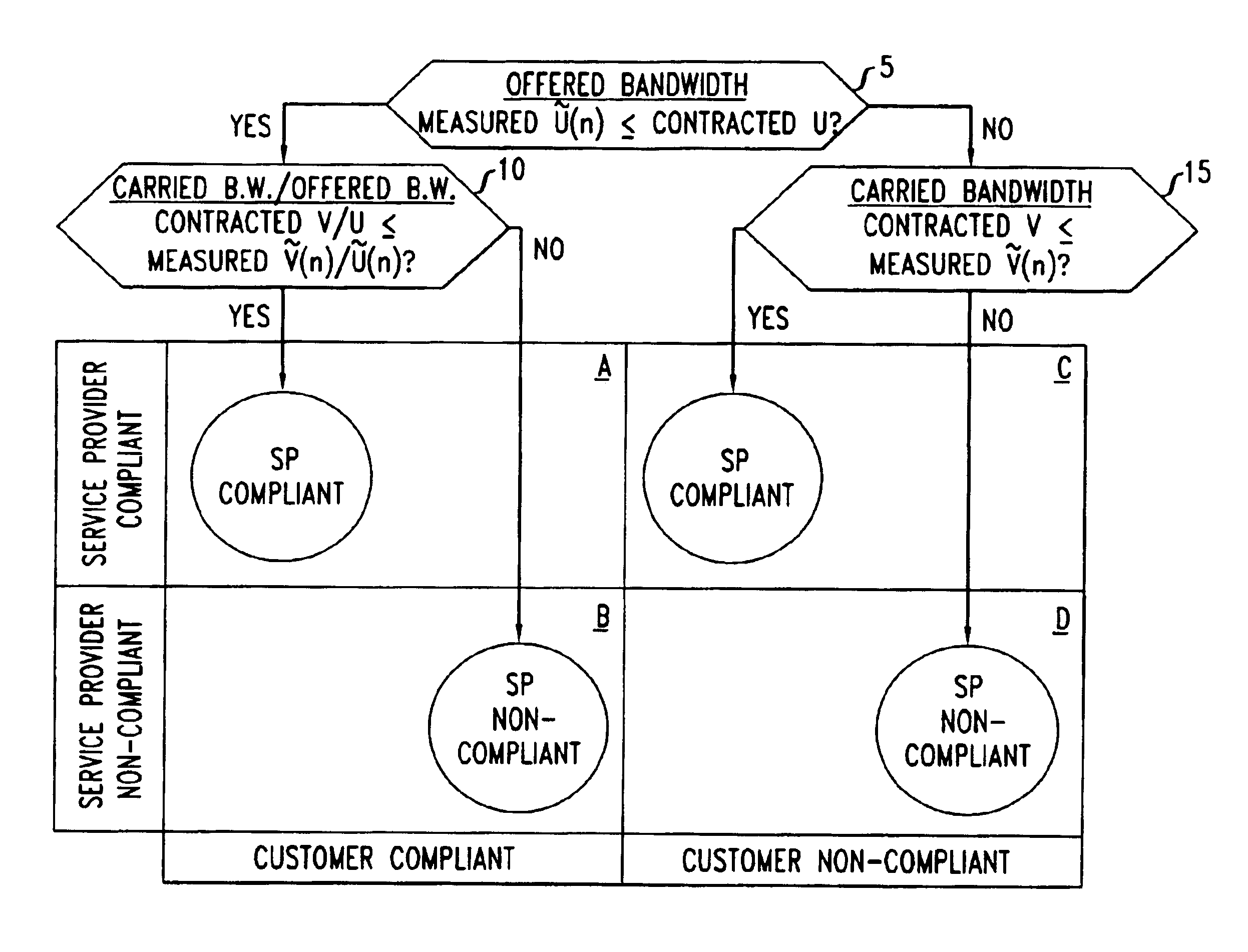
InactiveUS20030101263A1Data processing applicationsMetering/charging/biilling arrangementsPacket communicationNon compliance
Disclosed are network management procedures that apply measurements of traffic load to achieve greater efficiency in the operation of the network. In a method for deciding whether to route an incoming call on a selected potential service route, the potential service route is treated preferentially if each of its links has available capacity that is more than sufficient by a specified margin. In a method for computing billing revenues, the non-compliance of the network service provider with contracted requirements for carried load causes a revenue penalty to be exacted for lost bandwidth.
Owner:WSOU INVESTMENTS LLC
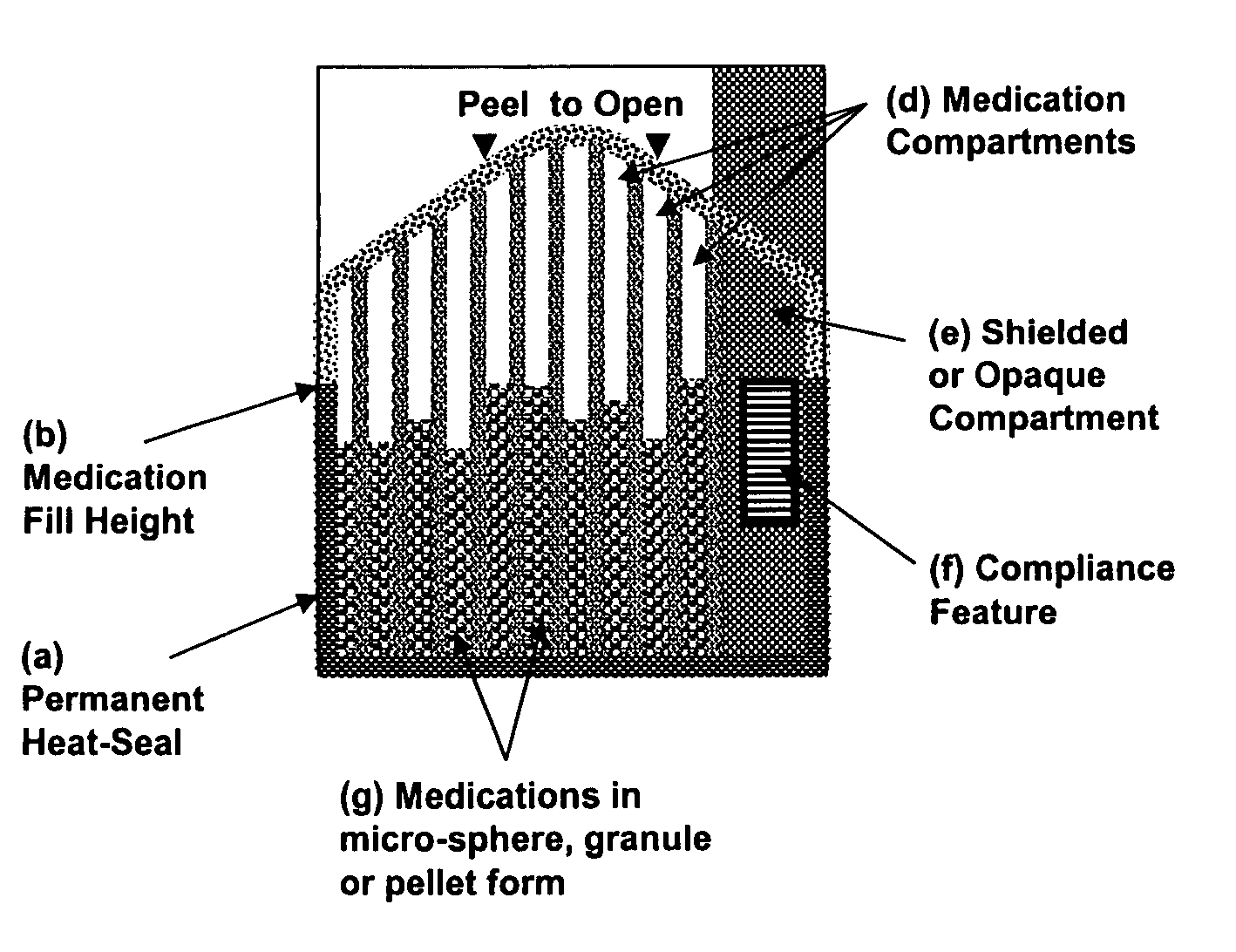
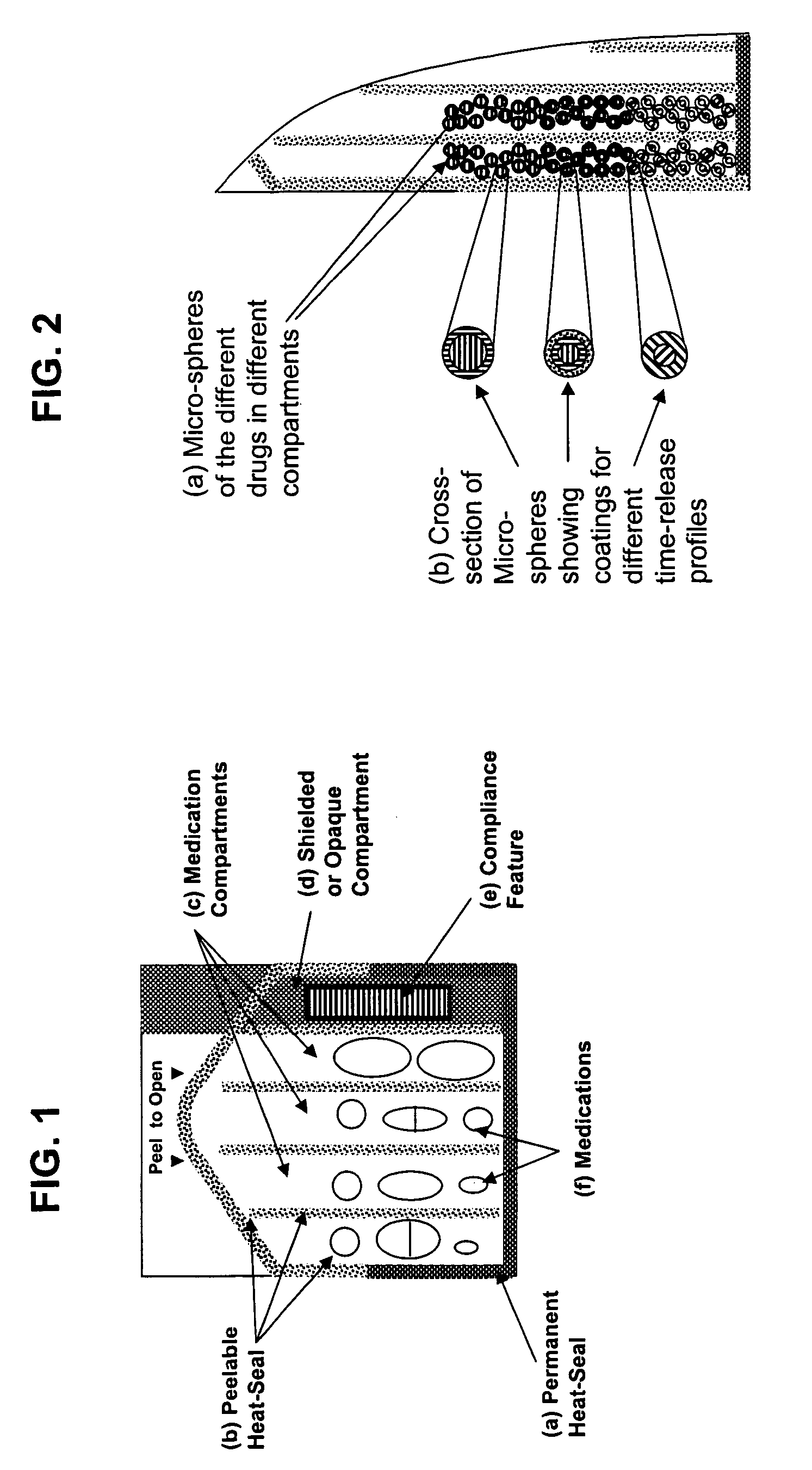
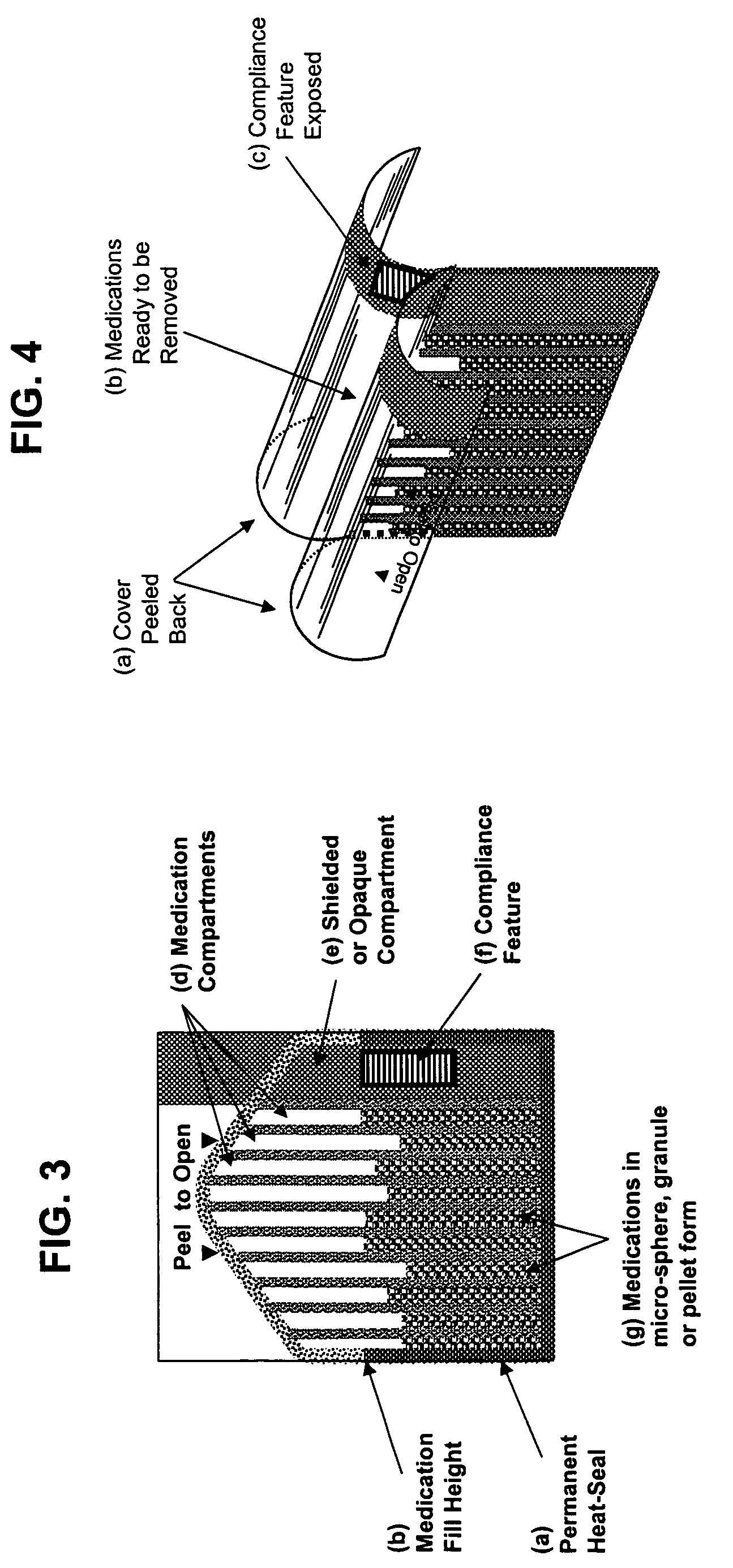
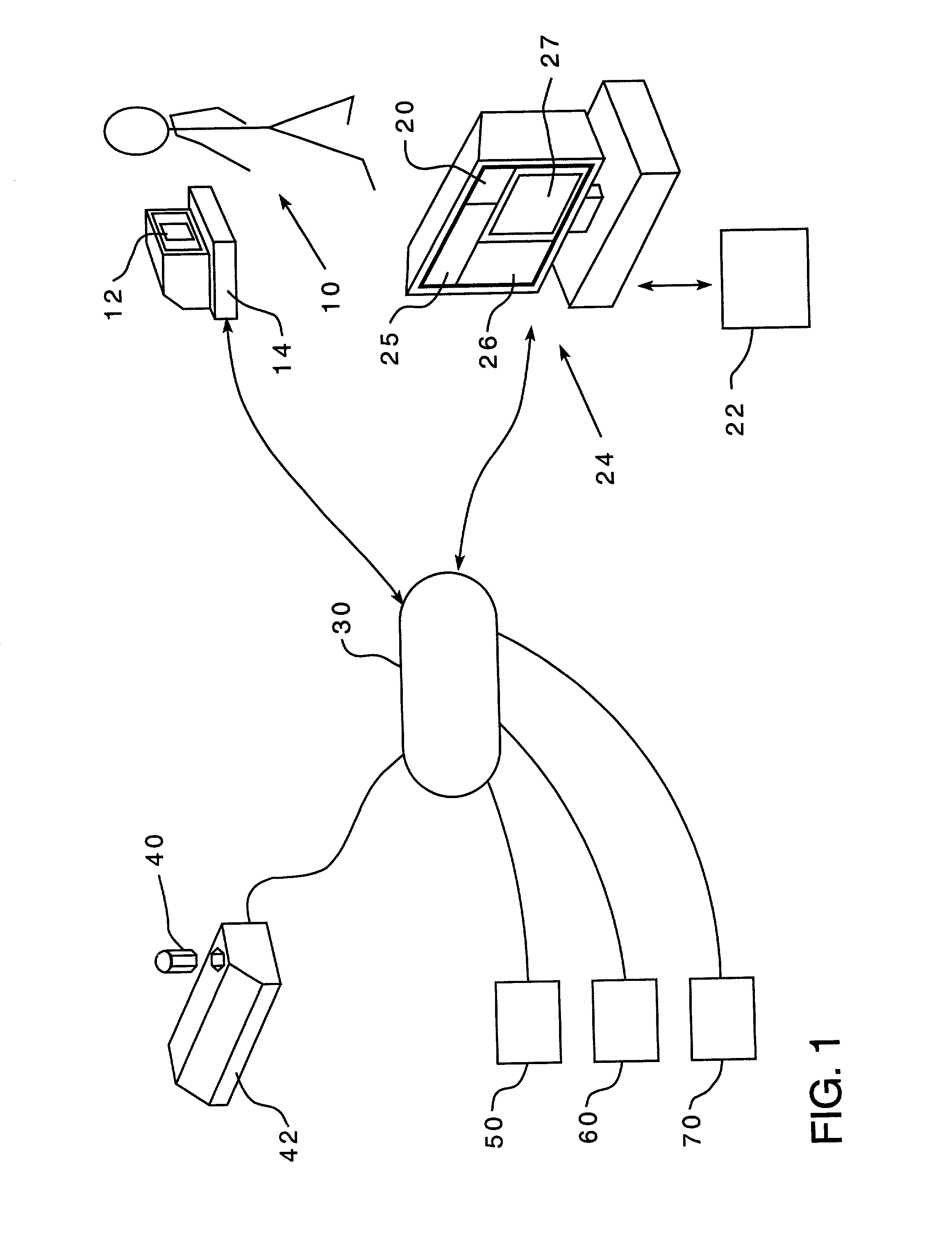
Unified ingestion package and process for patient compliance with prescribed medication regimen
Techniques for reducing non-compliance of medication use are described. The techniques involve generating and storing an identifier for a specific dosage instance of a specific patient, and creating a package that includes a mechanism for conveying the identifier. Once the package is created, a set of one or more medications that are prescribed to be taken by the specific patient at the specific dosage instance are placed in the package. The set of medications within the package in a manner that prevents the identifier from being perceived from the mechanism until the package is opened. Once opened, the identifier is perceived and communicated back to an automated compliance system.
Owner:RAMASUBRAMANIAN NARAYANAN +1
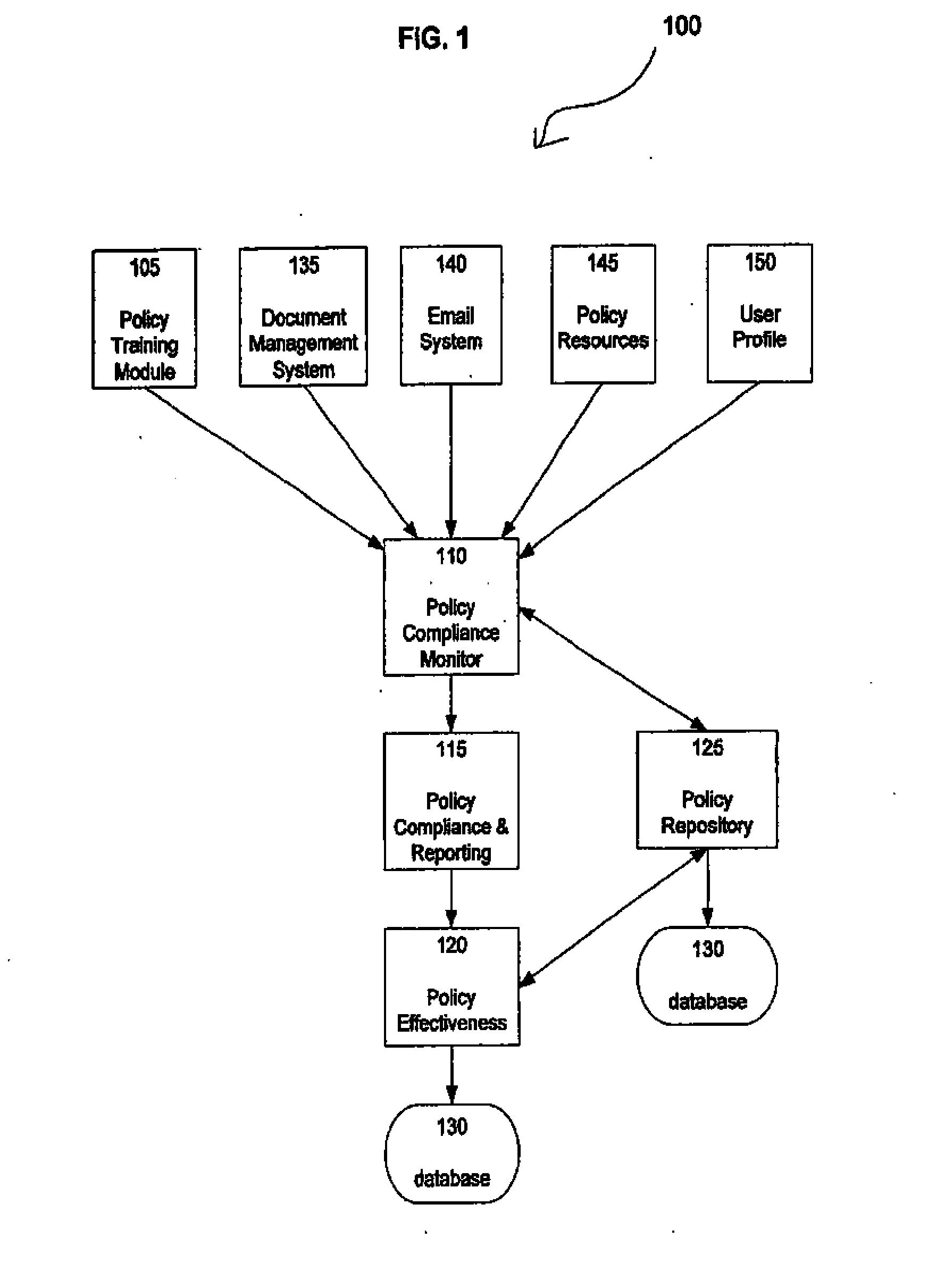
Network Policy Management And Effectiveness System
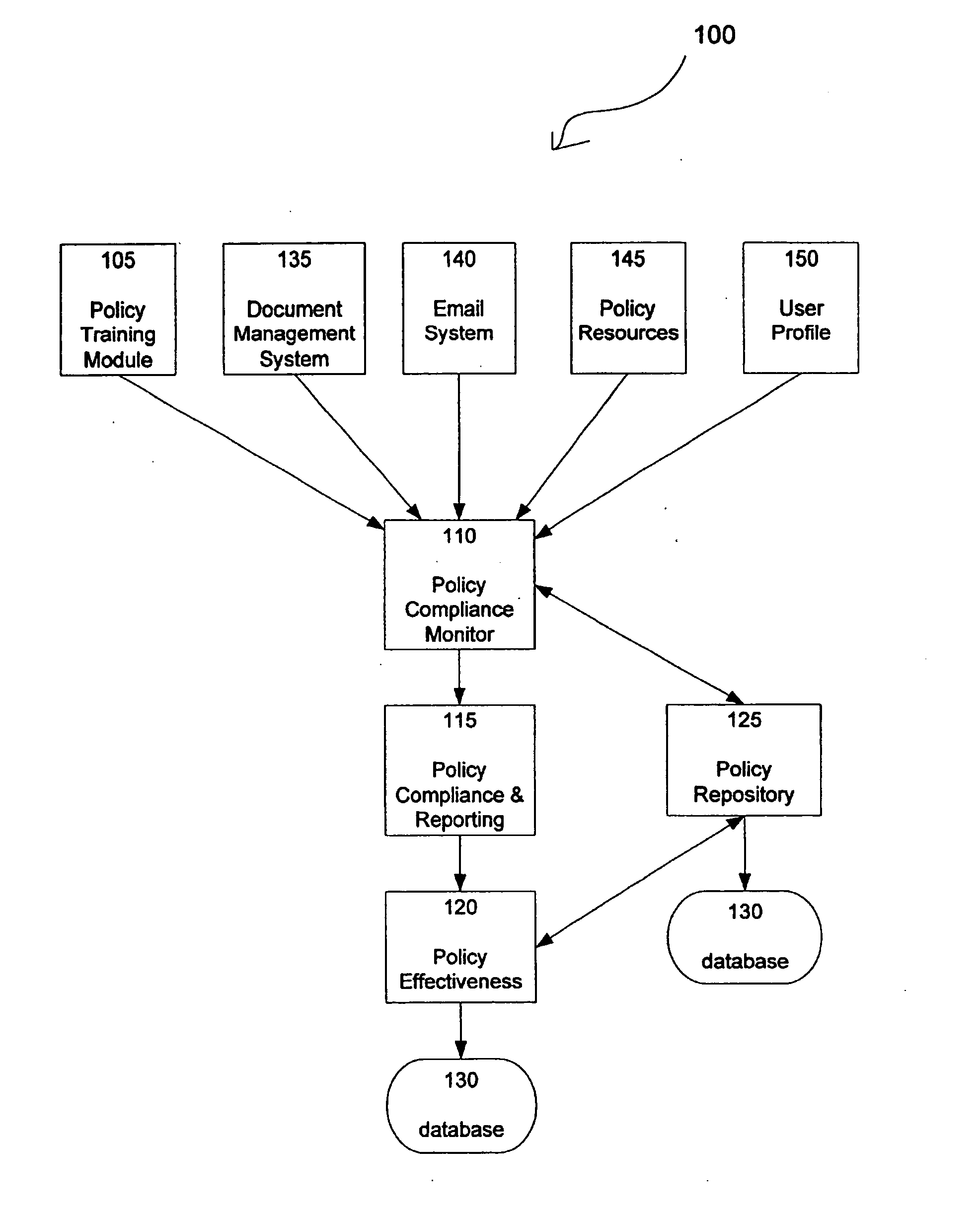
InactiveUS20070261121A1Digital data processing detailsError detection/correctionNon complianceNetwork security policy
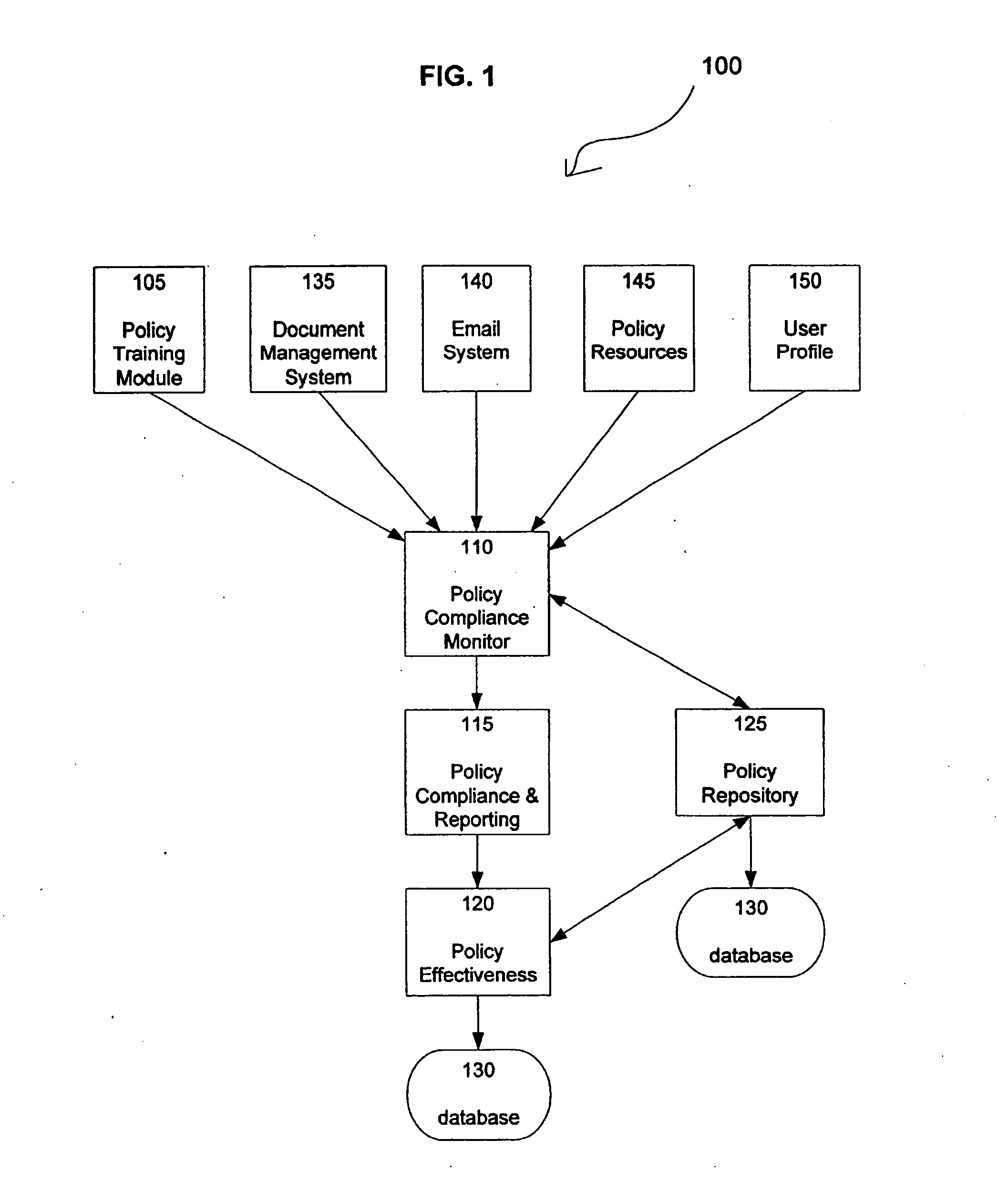
The Present Invention discloses a method and apparatus for maintaining policy compliance on a computer network. A system in accordance with the principles of the Present Invention performs the steps of electronically monitoring network user compliance with a network security policy stored in a database, electronically evaluating network security policy compliance based on network user compliance and electronically undertaking a network policy compliance action in response to network security policy non-compliance. The network policy compliance actions may include automatically implementing a different network security policy selected from network security policies stored in the database, generating policy effectiveness reports and providing a retraining module to network users.
Owner:LONGHORN HD LLC
Network resource configurations
ActiveUS20120290715A1Improve performanceSave capacityDigital computer detailsHybrid transportTime rangeNon compliance
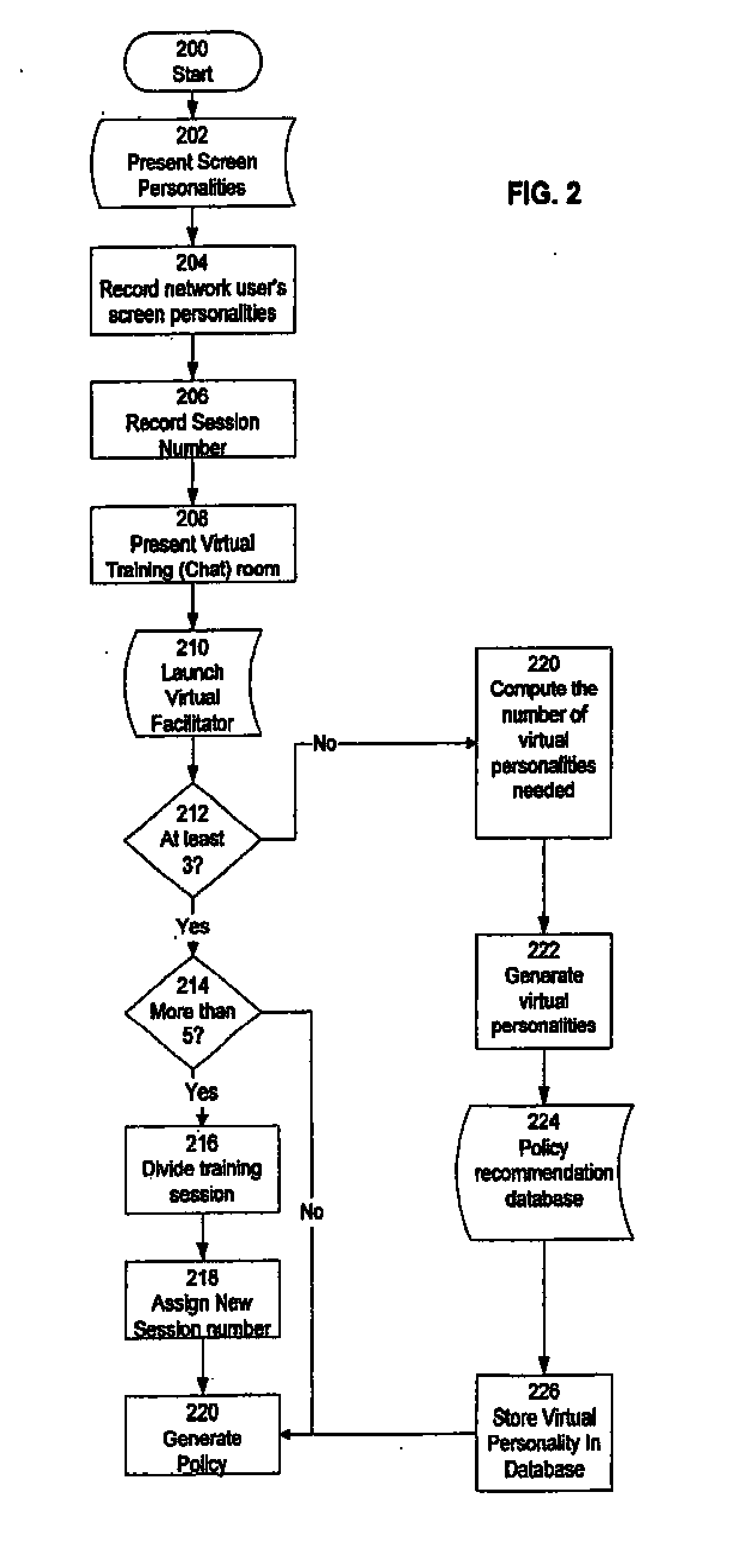
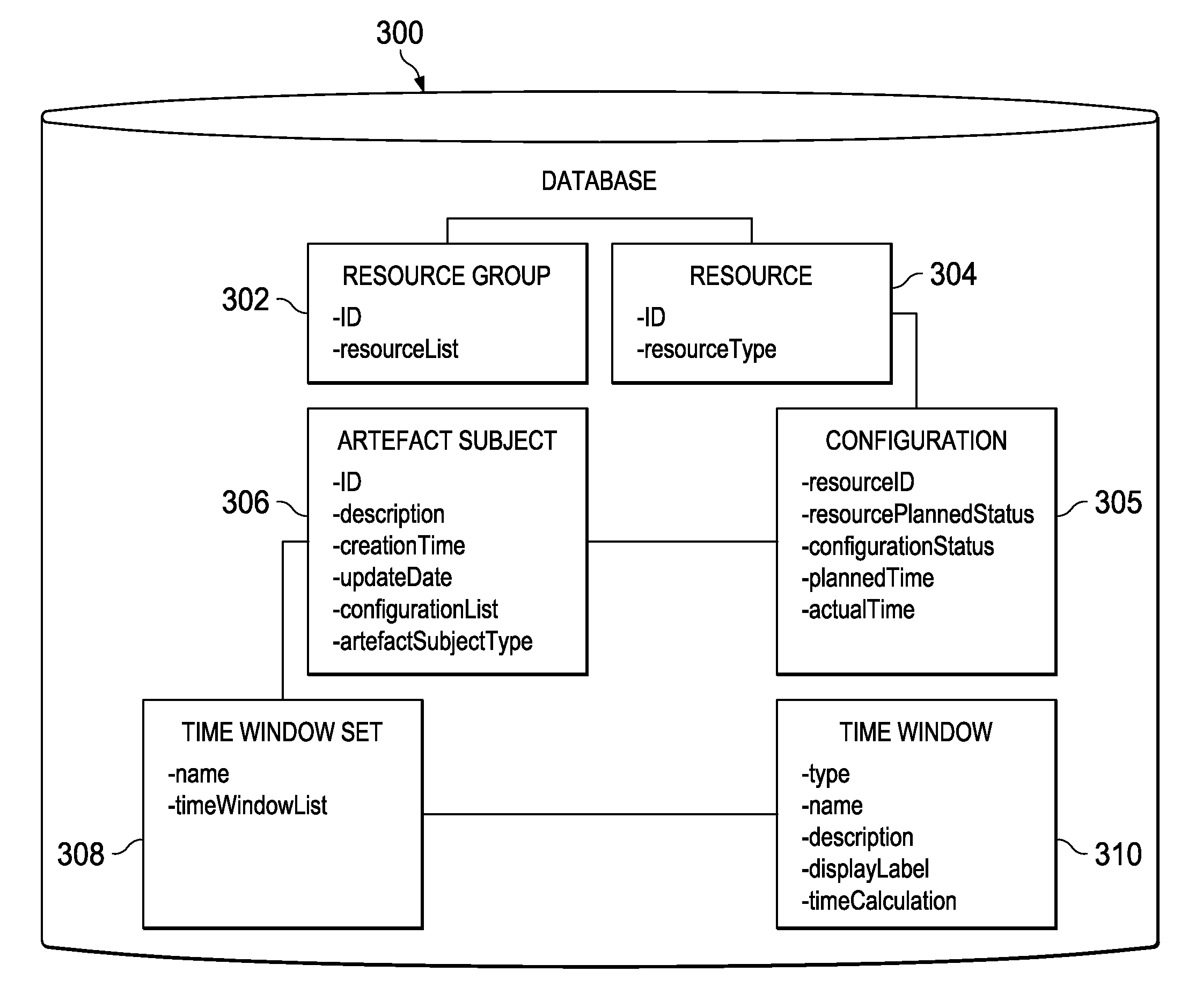
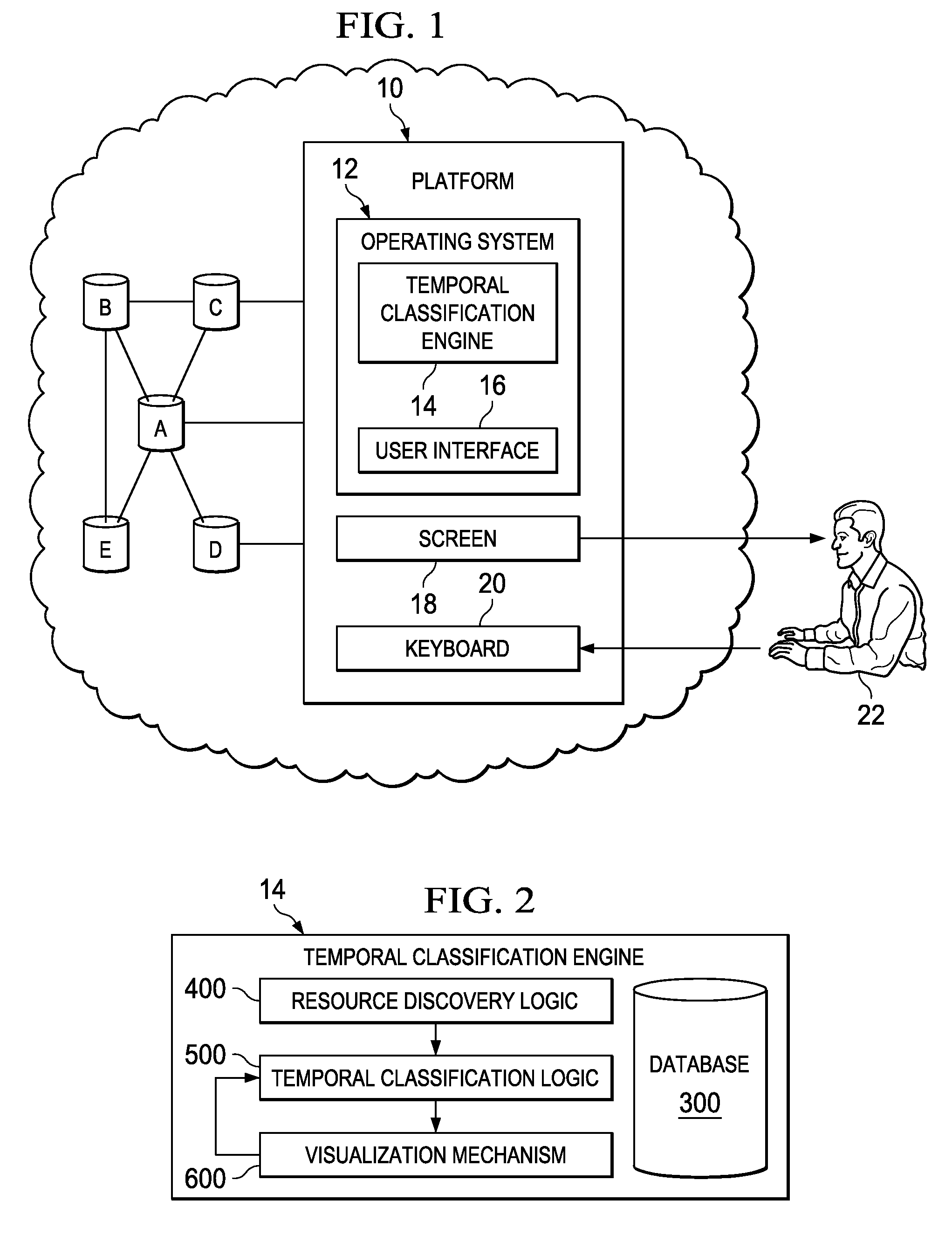
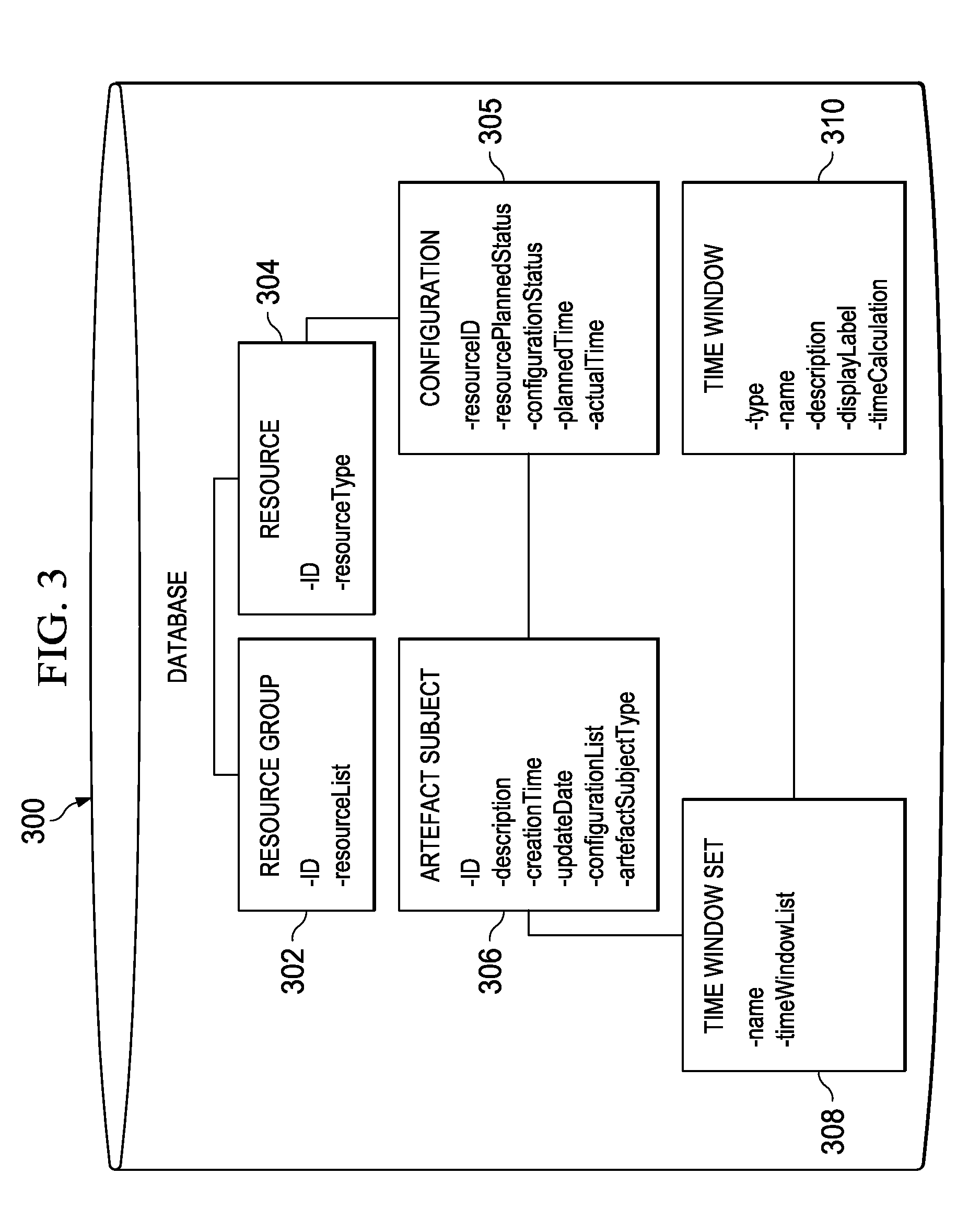
Temporally classifying and visually representing network and IT infrastructure with planned or occurred configuration activities and / or policy compliance or non-compliance of network and IT resources, including a method, apparatus and computer program for gathering and classifying observable configuration aspects of resources and relationships in a network of resources, wherein the method comprises: analyzing the network of resources to collect planned configuration times and actual resource status; monitoring actual resource status to make determinations if planned configurations are executed; and updating a network configuration status with the determinations. Planned and actual configurations are collected and monitored over a defined time range extending before and / or after a time zero. Each planned or actual configuration is categorized with respect to one or more time windows in the defined time range.
Owner:IBM CORP
Measurement-based management method for packet communication networks
InactiveUS6954739B1Metering/charging/biilling arrangementsComputer security arrangementsPacket communicationNon compliance
Owner:WSOU INVESTMENTS LLC +1
Network policy management and effectiveness system
InactiveUS20050010820A1Maintaining policy complianceMemory loss protectionDigital data processing detailsNetwork security policySecurity policy
A method, apparatus, and article of manufacture for maintaining policy compliance on a computer network is provided. The method provides the steps of electronically monitoring network user compliance with a network security policy stored in a database, electronically evaluating network security policy compliance based on network user compliance, and electronically undertaking a network policy compliance action in response to network security policy compliance.
Owner:LONGHORN HD LLC
Methods and systems for compliance monitoring knowledge base
ActiveUS7937319B2Low costQuality improvementDigital data information retrievalFinanceCompliance MonitoringData store
A knowledge base and methods for use in connection with a policy compliance monitoring system operative to determine exceptions to policies expressed by computer-executable policy statements. The system allows establishment, codification, and maintenance of enterprise policies, monitors electronic transactions of the enterprise from various and possibly heterogeneous data sources, detects exceptions to established policies, reports exceptions to authorized users such as managers and auditors, and / or provides a case management system for tracking exceptions and their underlying transactions. The knowledge base comprises extractor files that are utilized for extracting information from data sources for utilization in policy compliance monitoring, a mapper for normalizing data from the data sources against a system ontology and storing normalized data in a monitoring database, and computer-executable compliance policy statements used by a transaction analysis engine. The policy statements represent predetermined policies of the enterprise that apply to data stored in the monitoring database.
Owner:OVERSIGHT SYST INC
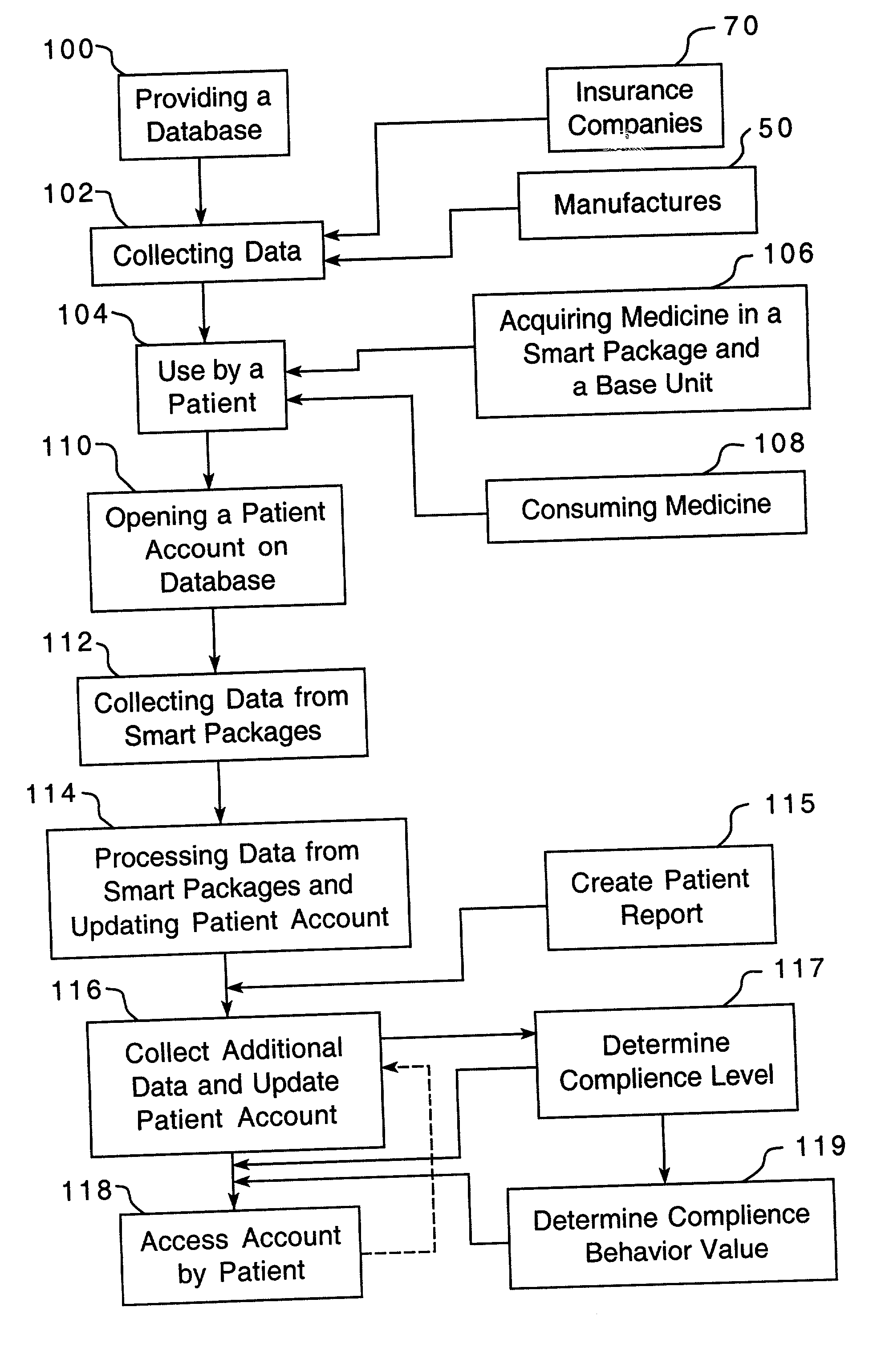
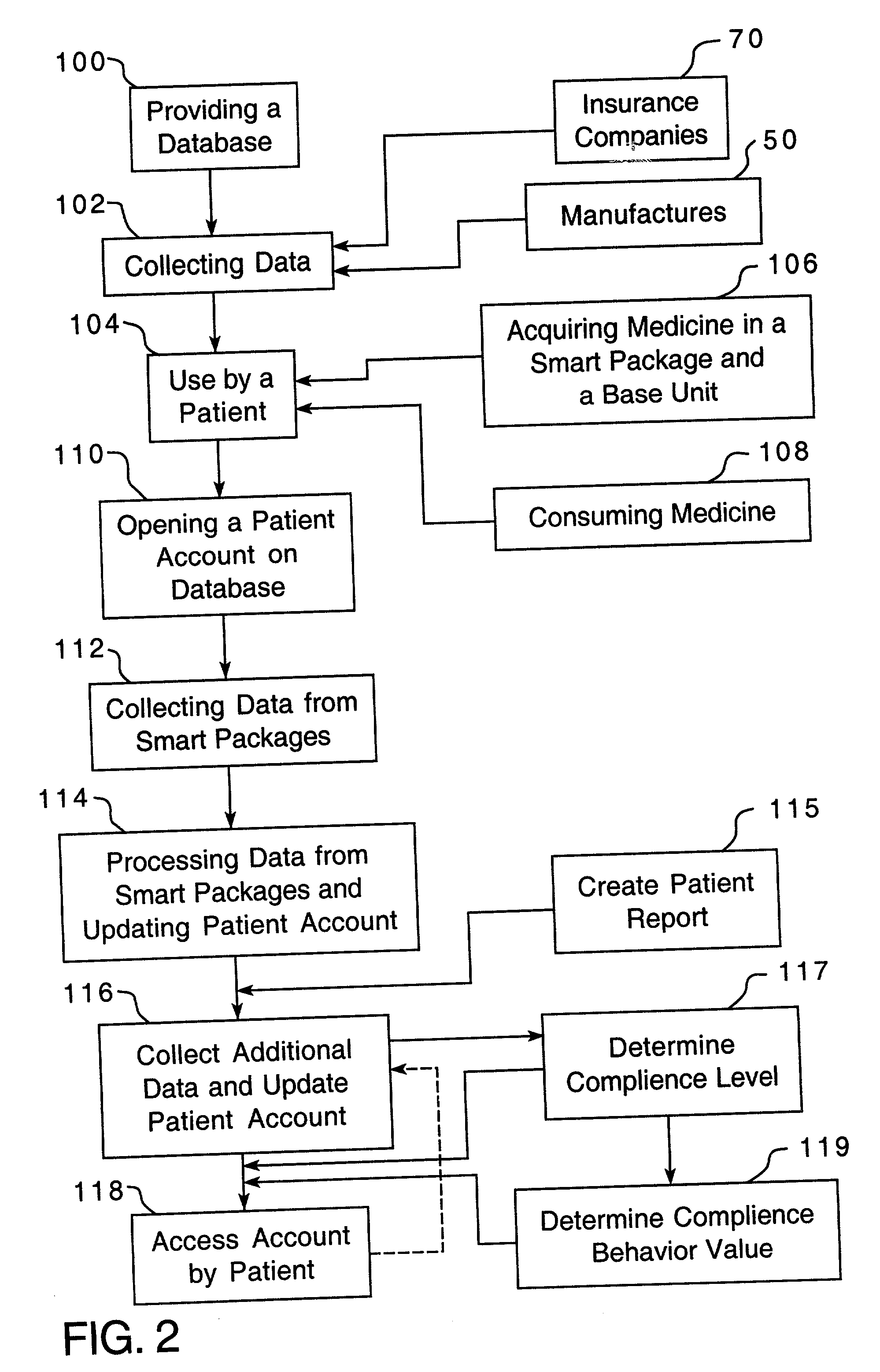
Method of providing comprehensive drug compliance information
A method is provided for encouraging patient compliance with a medicine regimen that includes interaction with the patient. The method includes the steps of providing a database which includes information regarding each patient. Information is supplied to the database by smart packages and other stakeholders. The information in the database is used to construct a patient report that may be viewed by the patient and others. Information in the patient report includes data on the patient's compliance, side effects of the medicine, expected results, interactions with other medicines, statements complimenting compliance, and warnings discouraging non-compliance. The patient report may be accessed by a common communication means such as the Internet.
Owner:CLEMENTI & ASSOCS
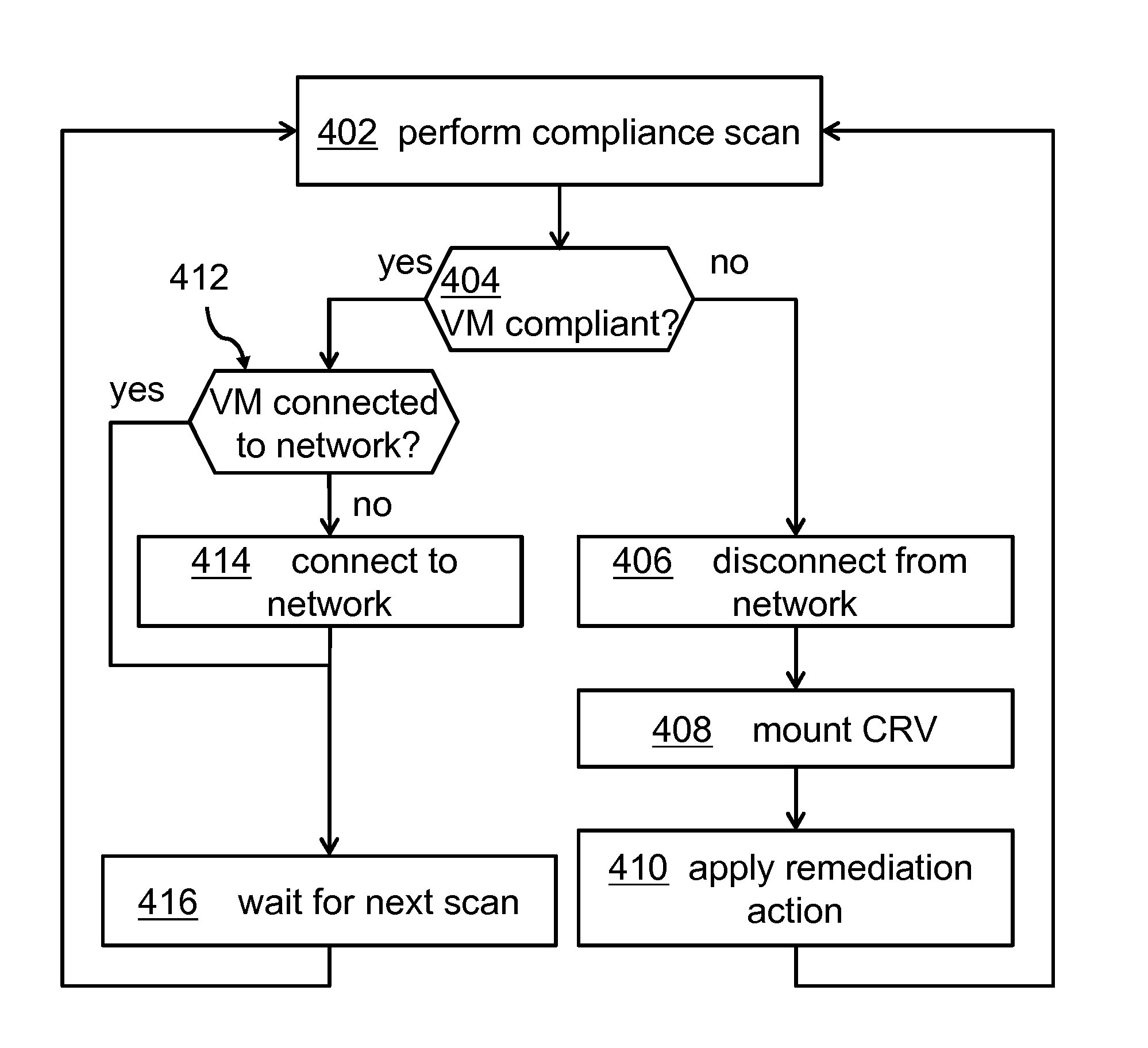
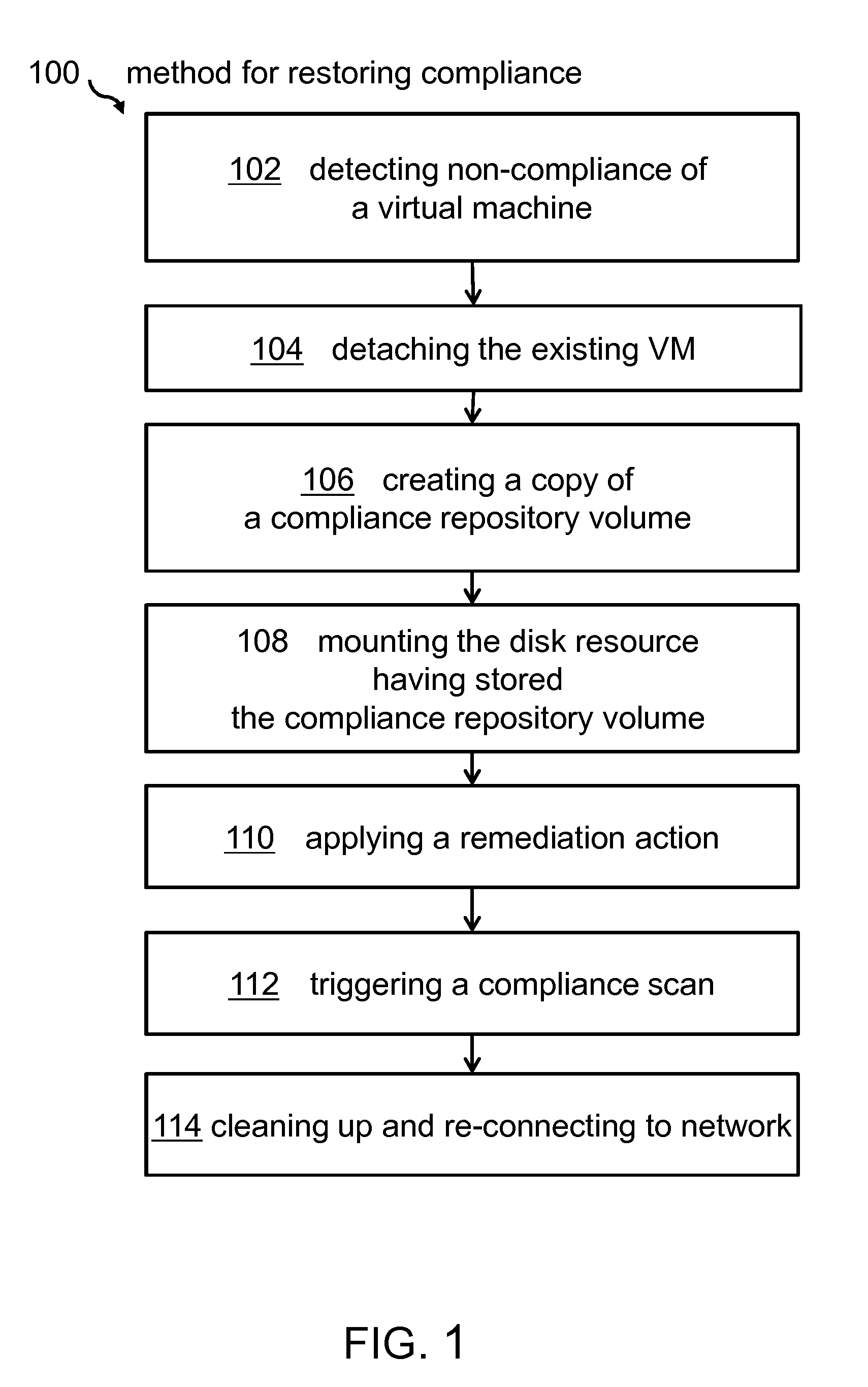
Managing Virtual Machine Policy Compliance
InactiveUS20150012920A1Improve system securityInfluence copying processSoftware simulation/interpretation/emulationMemory systemsNon complianceVirtual machine
A method for managing virtual machine policy compliance. The method for restoring compliance of a virtual machine found to be non-compliant to a compliance rule may comprise detecting non-compliance of a virtual machine using a compliance agent, detaching the virtual machine from a network, creating a copy of a compliance repository volume, mounting the newly requested disk resource having stored the copy of the compliance repository volume, applying a remediation action to the virtual machine, and triggering by the compliance agent a compliance scan for ensuring that the virtual machine complies to the compliance rule.
Owner:IBM CORP
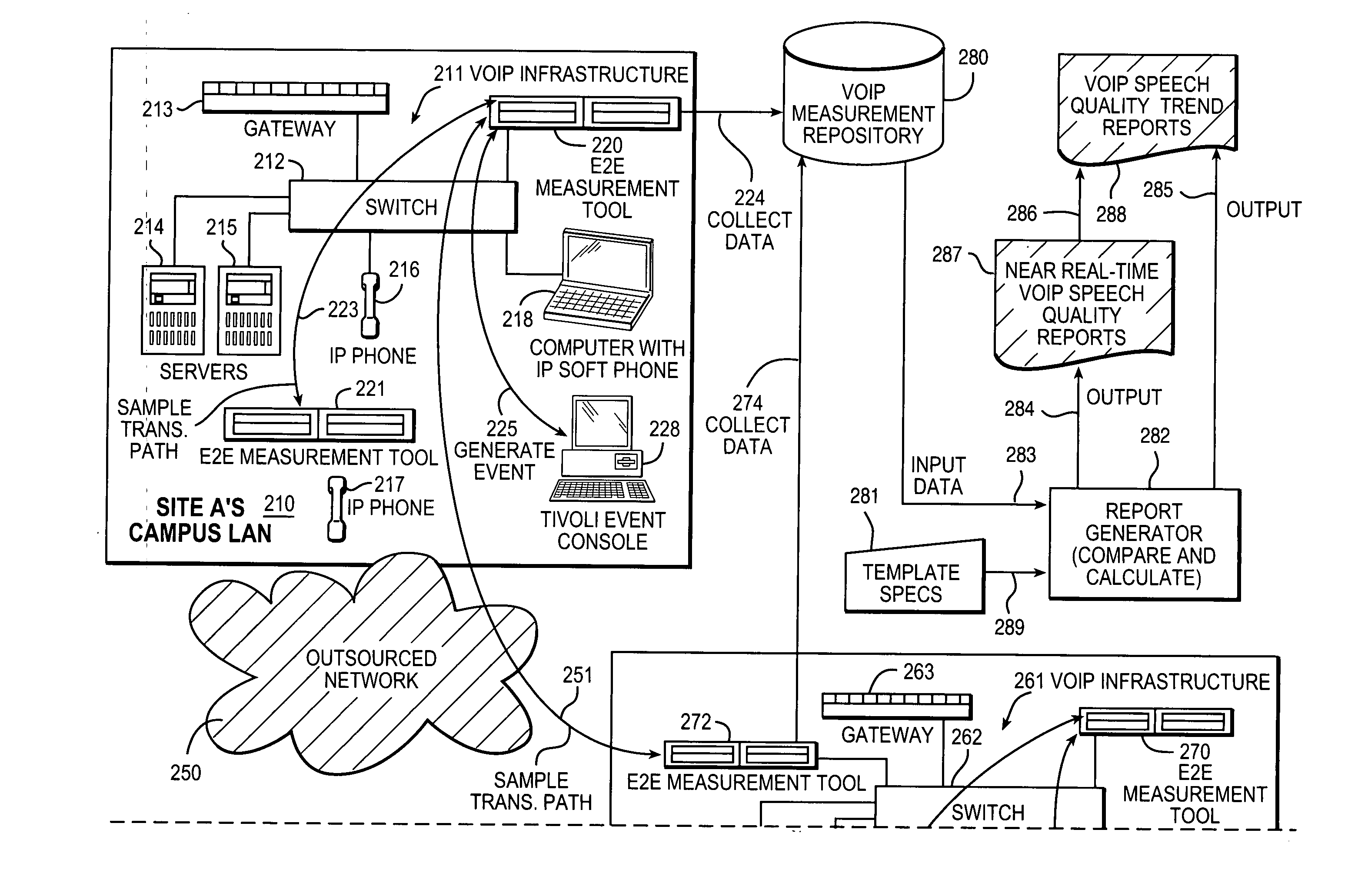
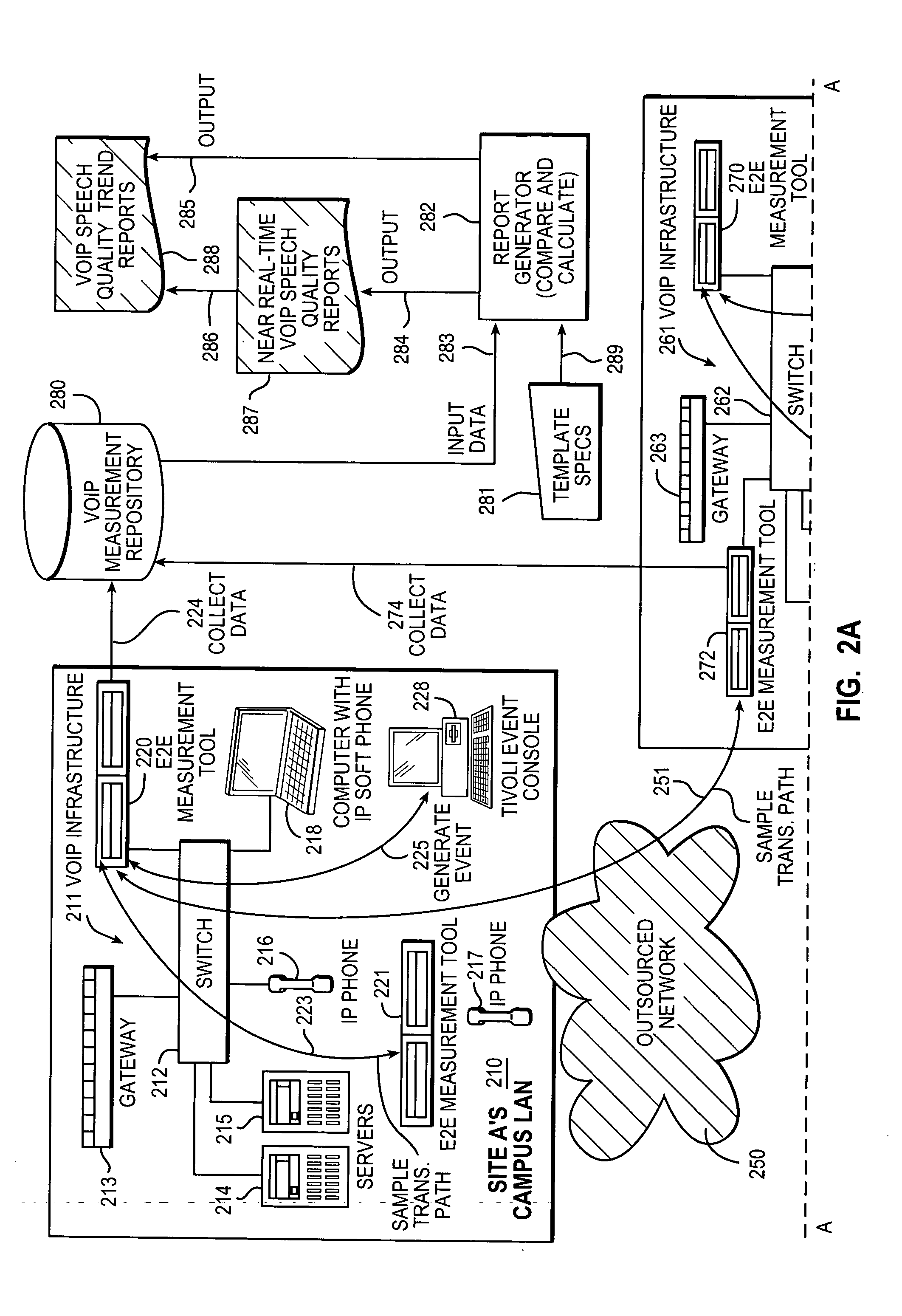
Measurement, reporting, and management of quality of service for a real-time communication application in a network environment
An example of a solution provided here comprises providing a measurement process including: (a) transmitting a test stream over a transmission path; and (b) measuring a quality-of-service indicator for a real-time communication application based on the transmitting; utilizing the measurement process, in continuously sampling a plurality of transmission paths in the real-time communication application's production environment; collecting data from the measurement process; comparing measured values to a threshold value; outputting a representation of compliance or non-compliance with the threshold value; and outputting a trend report based on the data; whereby the real-time communication application may be managed with reference to the threshold value. Such a solution may be selected for a Voice-over-Internet-Protocol application, a video conference application, or a speech-recognition application, to give some non-exclusive examples. One such example comprises measuring a speech-quality indicator for a Voice-over-Internet-Protocol application.
Owner:IBM CORP
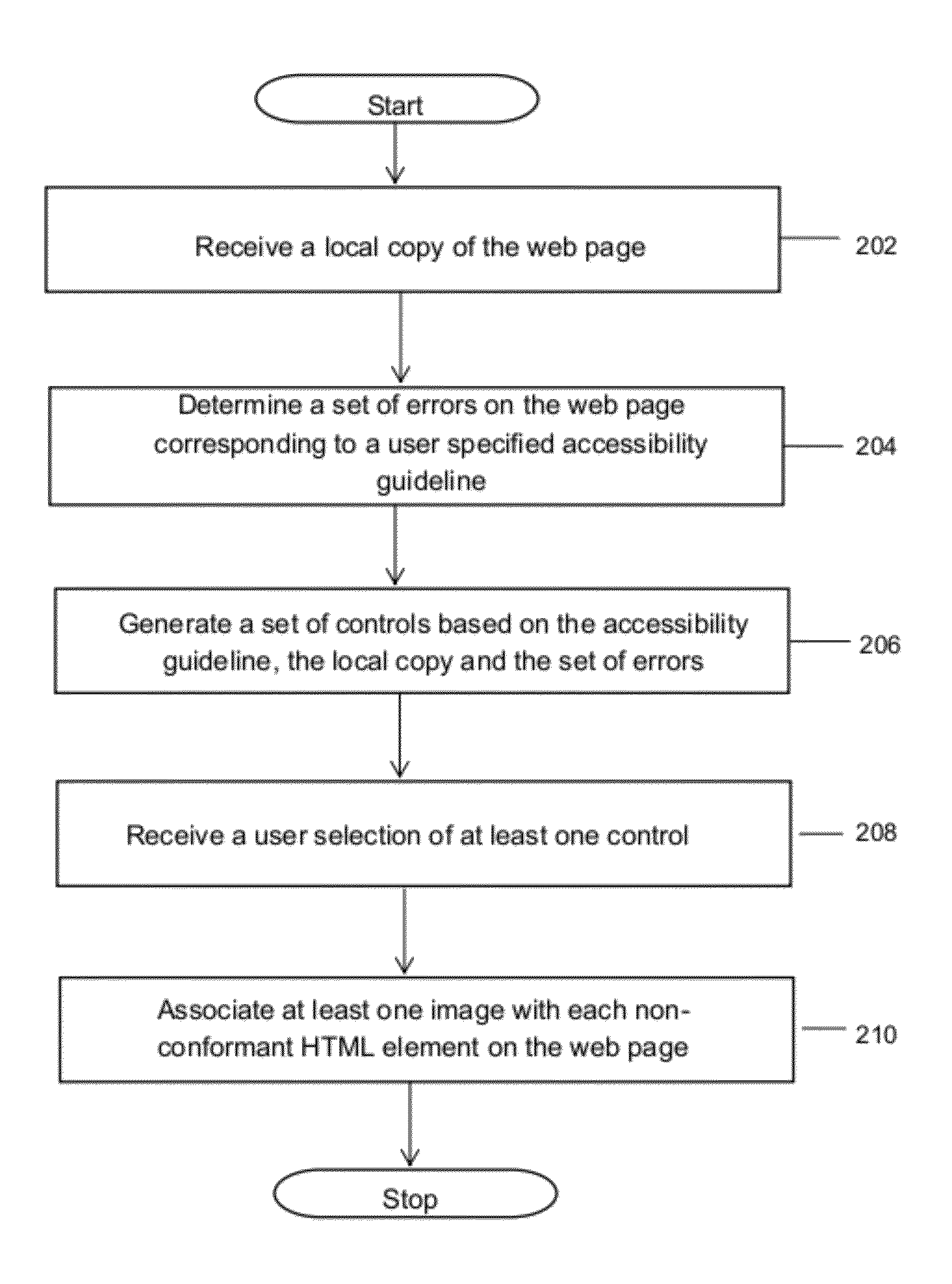
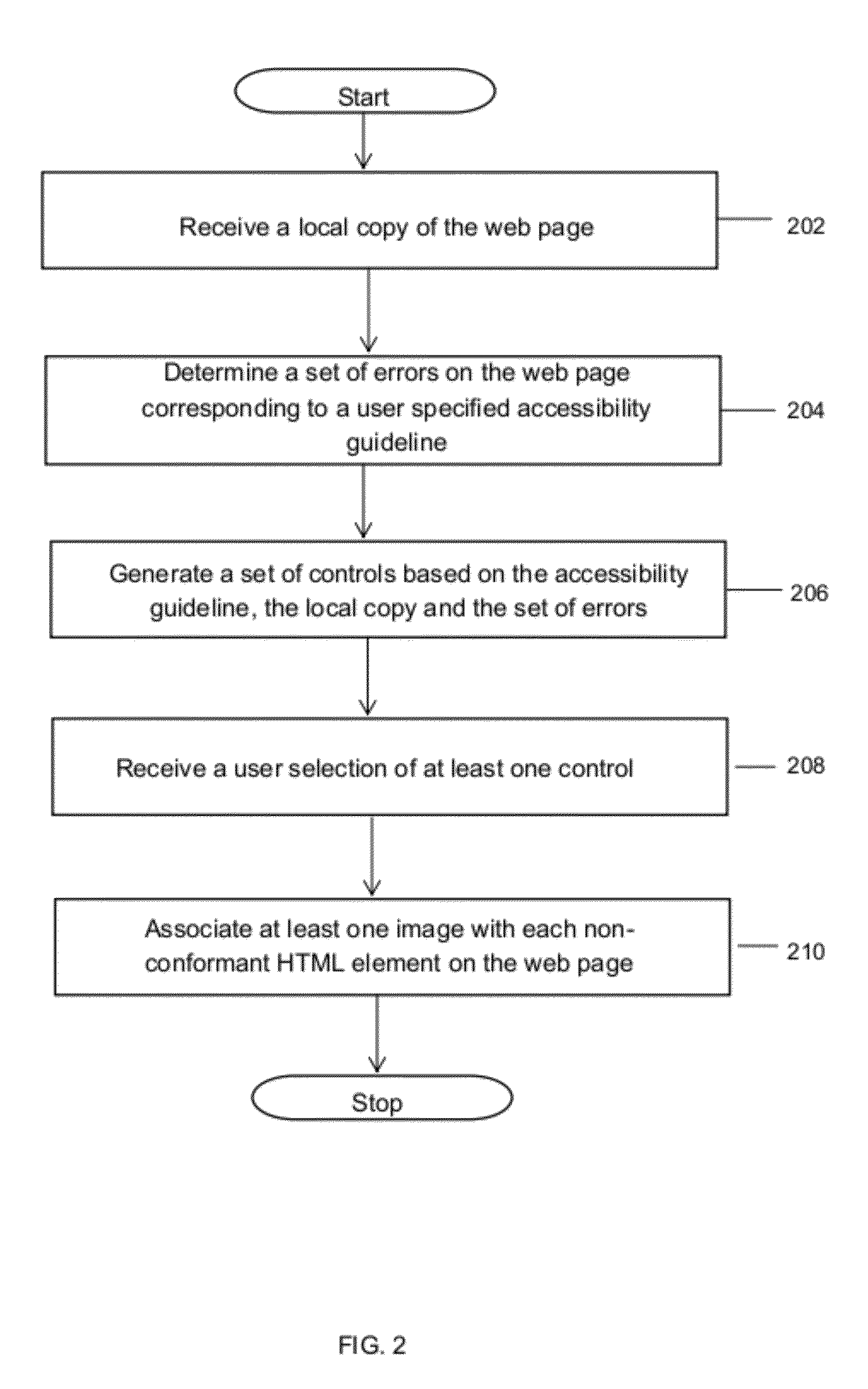
Method and system for reporting web standard non-compliance of web pages
ActiveUS20120254723A1Natural language data processingSpecial data processing applicationsNon complianceGuideline
A method and system for reporting web standard non-compliance of web pages is provided. The method includes receiving a local copy of the web page to be tested for web standard non-compliance. The method further includes determining a set of errors on the web page corresponding to a user specified accessibility guideline. Thereafter an HTML overlay report is generated based on the determined set of errors. Further, a set of controls is generated based on the user specified accessibility guideline, the local copy and the set of errors. Finally, one or more controls are selected from the set of controls and based on the one or more selected controls and the determined set of errors one or more images are associated with each HTML element non-conformant to the accessibility guideline on the web page.
Owner:INFOSYS LTD
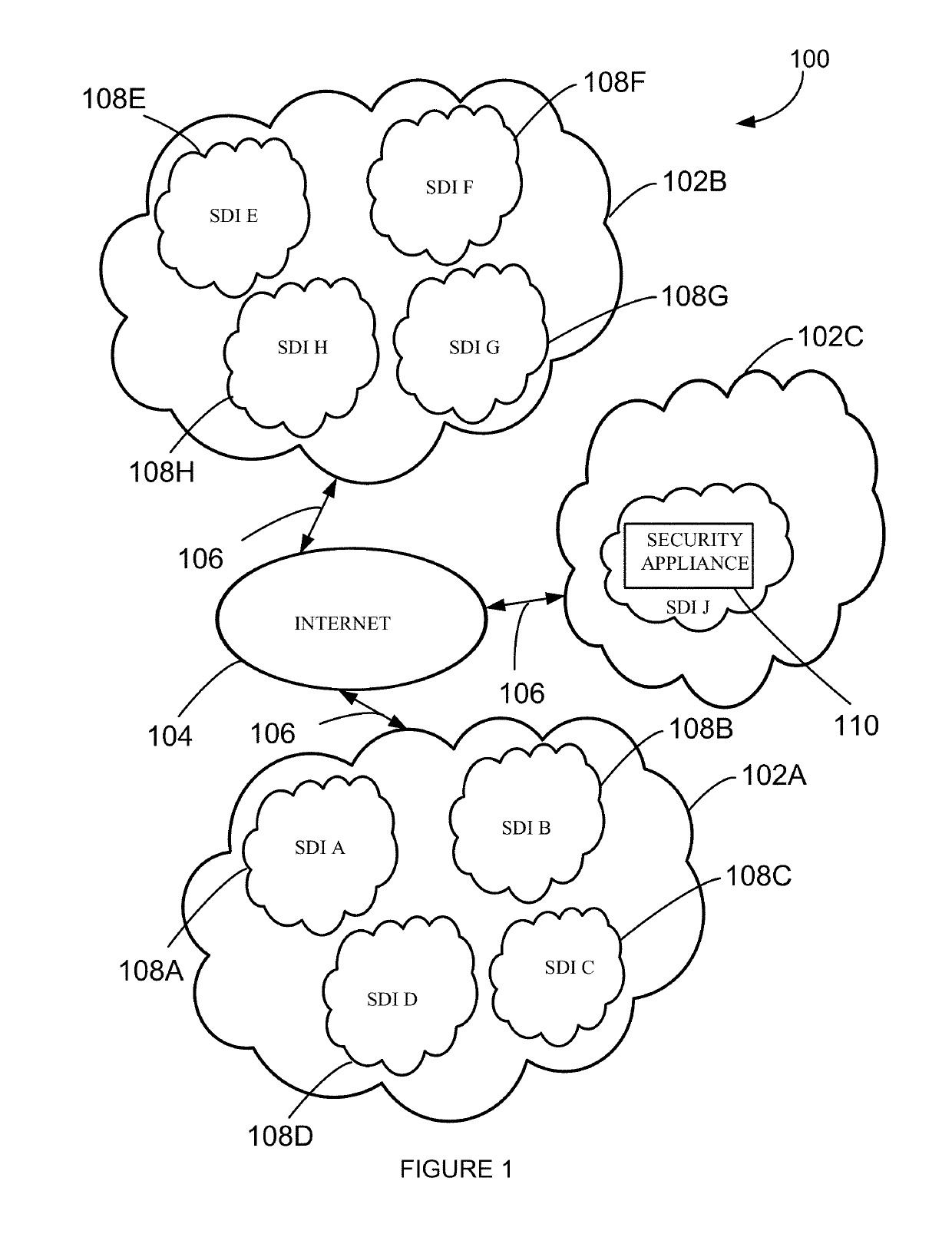
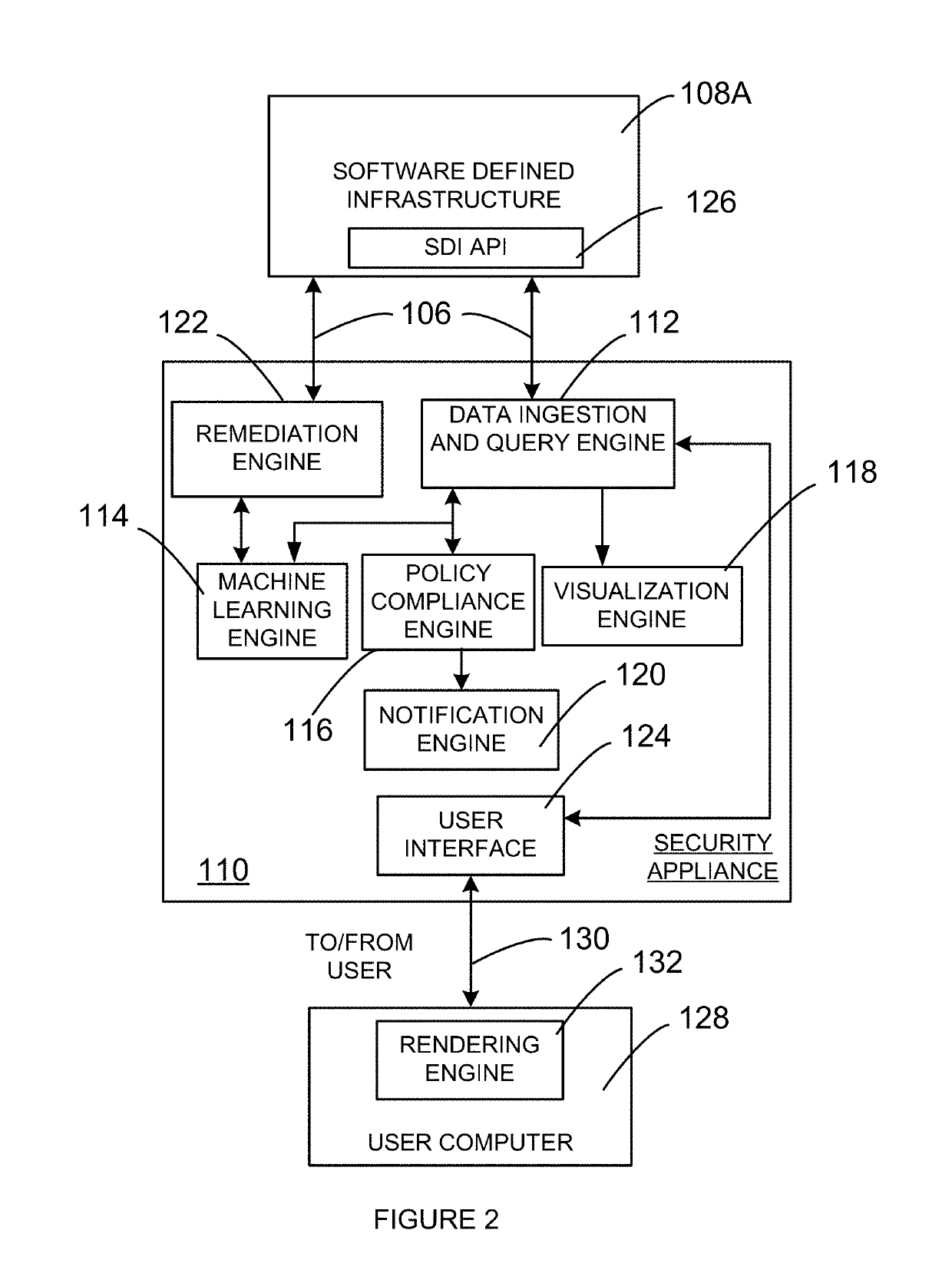
Security appliance to monitor networked computing environment
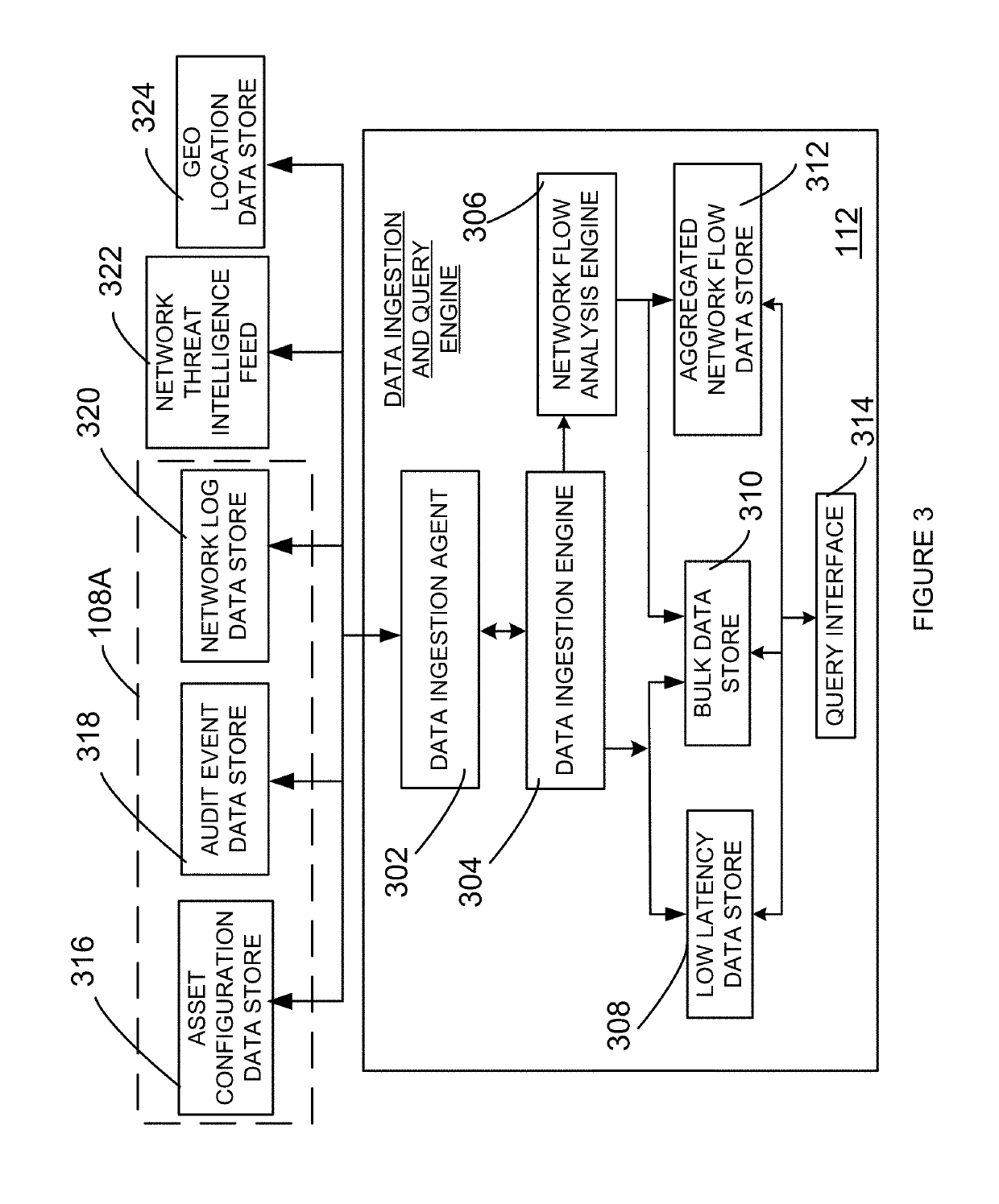
A system and method to evaluate a software defined infrastructure is disclosed. A security appliance is used to evaluate the software defined infrastructure. The security appliance includes a data ingestion and query engine. The data ingestion and query engine is configured to retrieve configuration and operational information associated with the software defined infrastructure, extract selective information from the retrieved configuration and operational information, and store extracted selective information in a plurality of data store. A policy compliance engine is configured to evaluate selectively stored information for compliance to a policy and generate a report based on the evaluation.
Owner:PALO ALTO NETWORKS INC
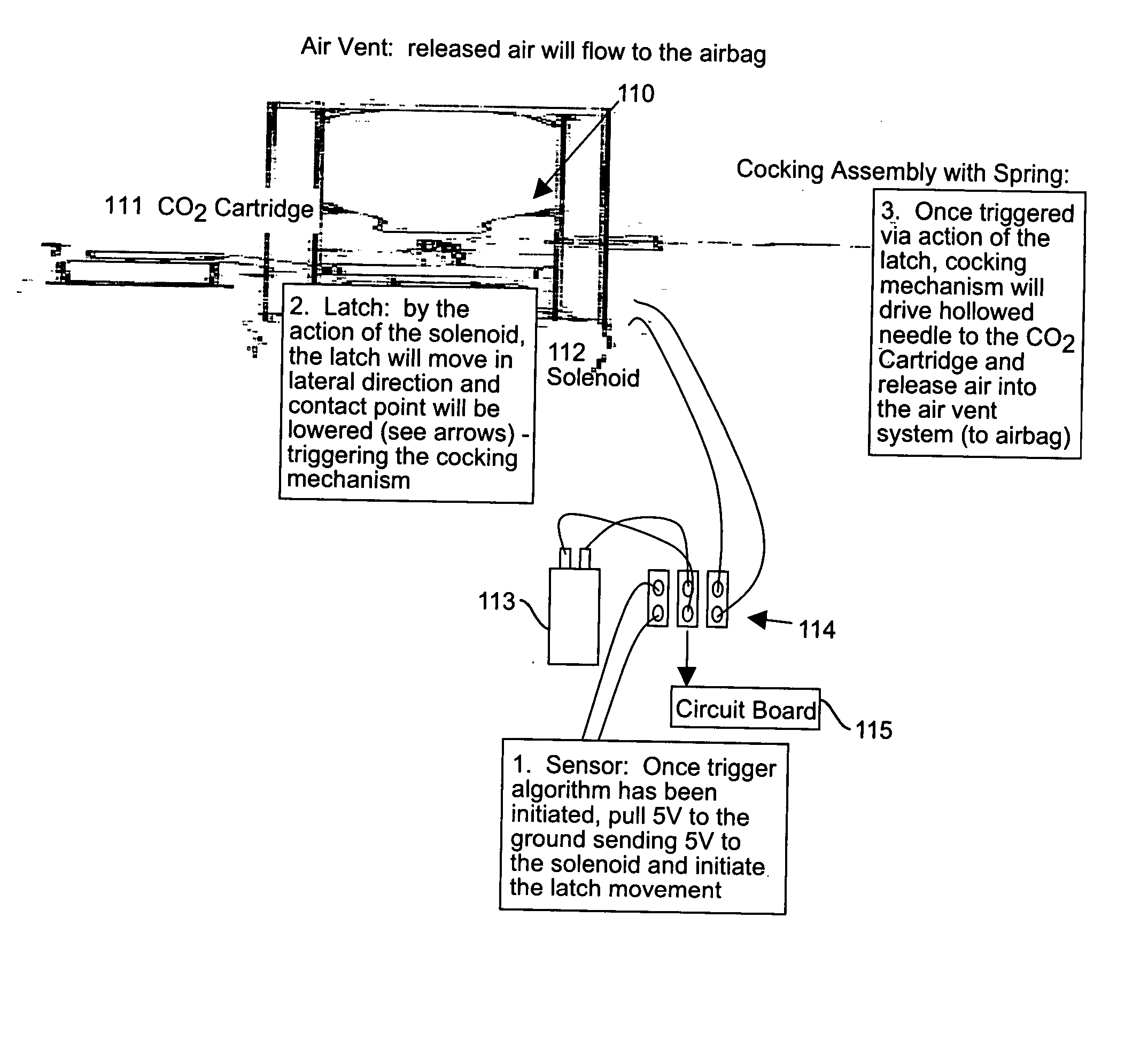
Fall-sensing systems, hip protector systems, and other protective systems
InactiveUS20060049950A1Small sizeInertial sensorsDiagnostic recording/measuringNon complianceHip protector
Fall-sensing systems are provided which do not generate false alarms for a non-falling event. Inventive fall-sensing systems may be magnetometer-free. Thin-profile (less than ½ inch, pre-inflation) wearable systems are provided. By removing the problem of false alarms and by slimming the pre-inflation profile, a practically useable wearable protection solution may be provided for an individual prone to falling. The number and severity of hip fractures in the elderly may be reduction. The inventive product actively assesses fall accidents and triggers an inflatable airbag protection device. The problem of non-compliance in wearing hip protectors that has previously limited the effectiveness of other hip protectors also has been solved.
Owner:VIRGINIA TECH INTPROP INC
Automated configuration of network devices administered by policy enforcement
ActiveUS8458301B1Guarantee support safetyDigital computer detailsTransmissionAuto-configurationComputer science
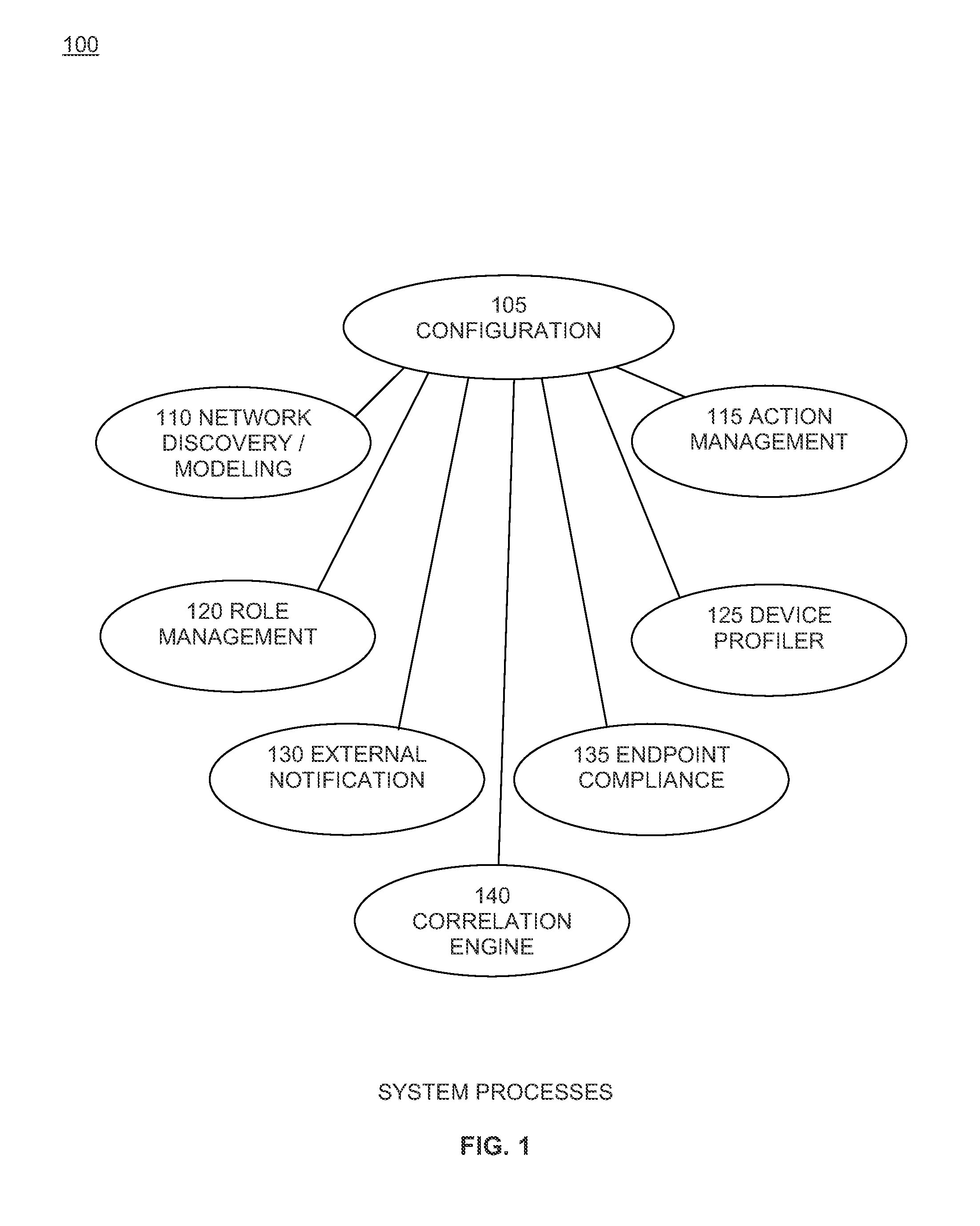
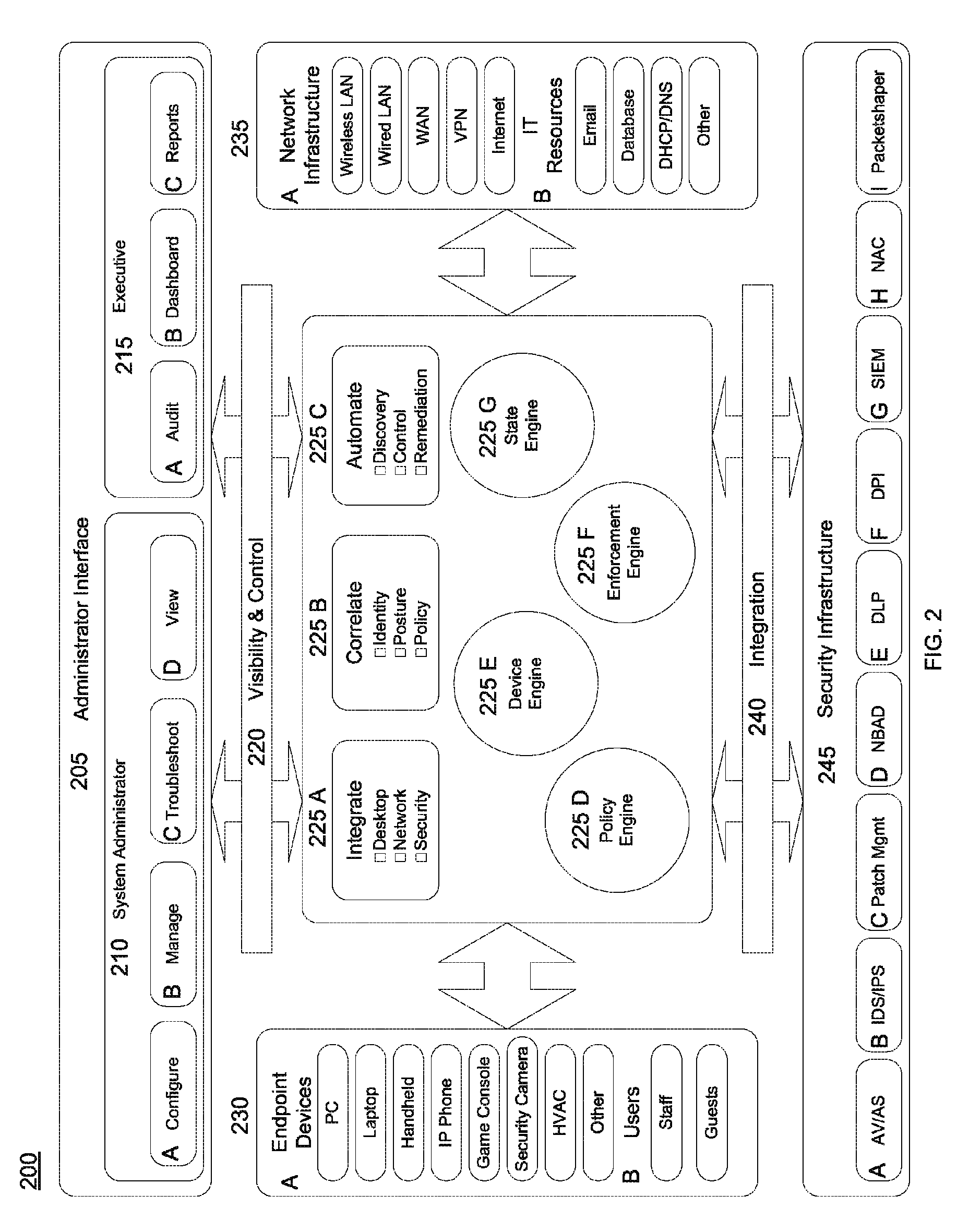
A system and method for dynamic device configuration enabling network and security administrators to define policies that indicate event and alert conditions within their networks. The policies incorporate information about network devices, endpoints connected to those devices, input from external security systems, local endpoint policy compliance, and date / time-of-day to determine whether to generate an event or alert. Events and alerts can be associated with actions that effect changes to network device configurations in order to maintain a desired operational state of the network.
Owner:FORTINET
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com