Long-acting non-water-carrier injection liquid and preparing method thereof
An injection and carrier technology, applied in the chemical field, can solve the problems of expensive equipment, difficult aseptic control, and larger particle size of aqueous nano-suspension, achieving less toxic and side effects, high sterility assurance factor, and improved physical stability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
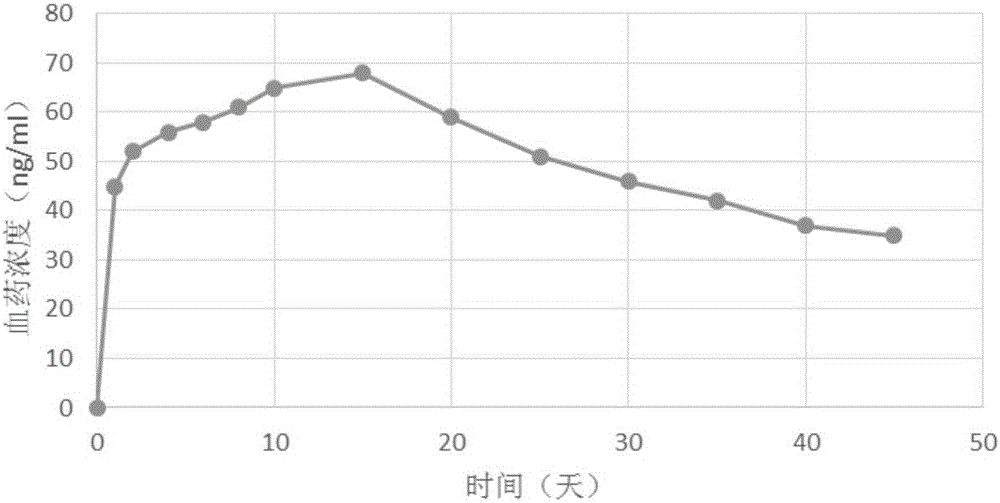
[0042] Embodiment 1 prepares lauroyl aripiprazole soybean oil injection
[0043] Specification: 295mg / ml
[0044] prescription:
[0045] name
purpose
Amount added
Aripiprazole Lauroyl
Main drug
380g
Soybean Oil for Injection
injection oil
1000g
Lecithin for Injection
Emulsifier
120g
Glycerin for Injection
osmotic regulator
210g
[0046]Technological process: Aripiprazole lauroyl is pulverized to a D90 of less than 50 microns with a jet mill (TC-30 superfine pulverization classifier, Beijing Sinopharm Longli Technology Co., Ltd.). Then take lauroyl aripiprazole 380g, soybean oil for injection 1000g, lecithin for injection 120g, glycerin for injection 210g, carry out primary dispersion with solid / liquid dispersion mixing system (PJ90 type, Shanghai Fluke Technology Development Co., Ltd.) , and then the sample was added to a high-pressure jet homogenizer (Nilu Solvay, Italy), and the inlet tempe...
Embodiment 2
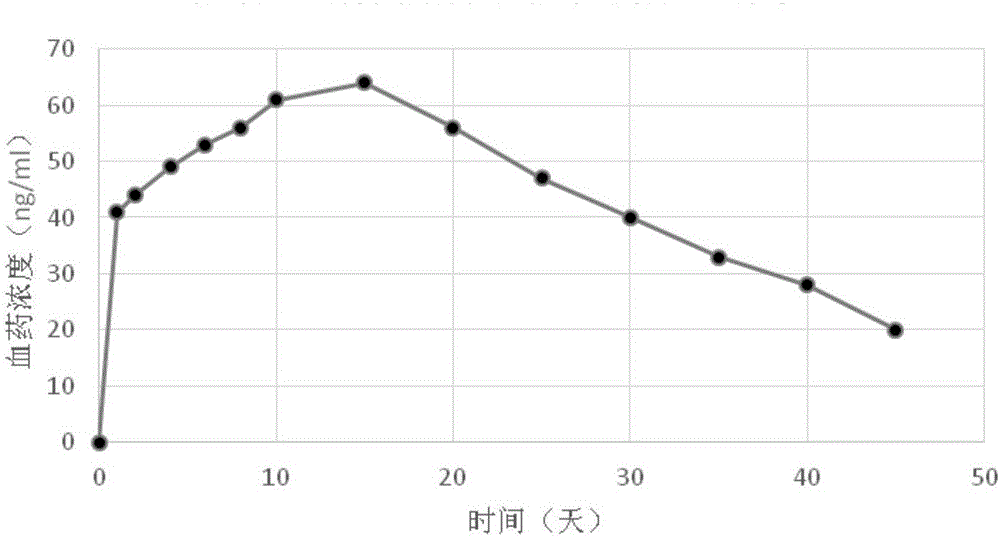
[0048] Embodiment 2 prepares paliperidone palmitate soybean oil injection
[0049] Specification: 156mg / ml
[0050] prescription:
[0051] name
purpose
Amount added
Paliperidone Palmitate
Main drug
203g
Soybean Oil for Injection
injection oil
1000g
Lecithin for Injection
Emulsifier
120g
Glycerin for Injection
osmotic regulator
210g
[0052] Technological process: pulverize paliperidone palmitate until the D90 is below 50 microns with a jet mill (TC-30 ultrafine pulverizer classifier, Beijing Sinopharm Longli Technology Co., Ltd.). Then get paliperidone palmitate 203g, soybean oil for injection 1000g, lecithin for injection 120g, glycerol 210g for injection, carry out primary with solid / liquid dispersion mixing system (PJ90 type, Shanghai Fluke Technology Development Co., Ltd.) Disperse, then join the sample in the high-pressure jet homogenizer (Italy Nilu Solvay Company), keep the inlet tempe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com