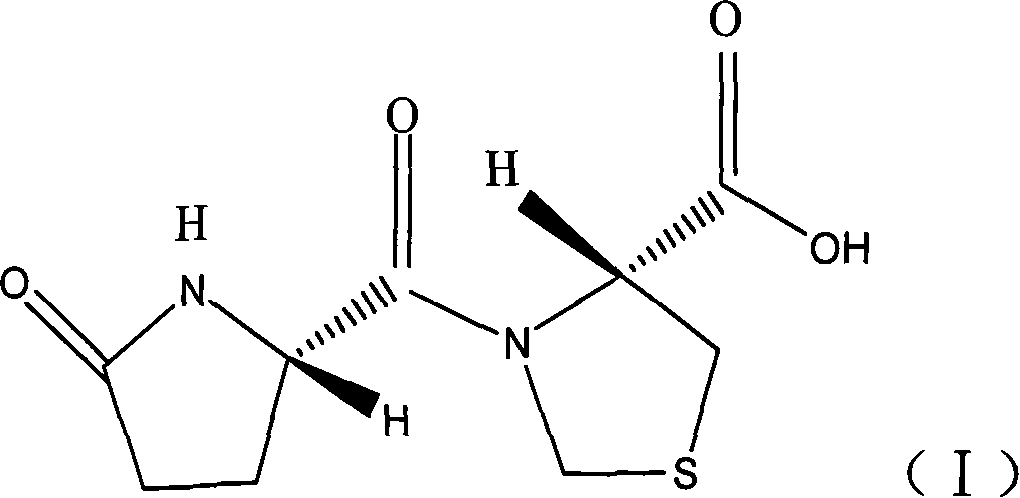
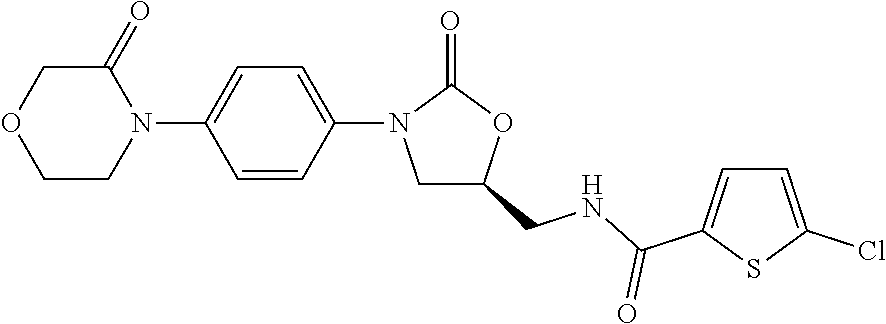
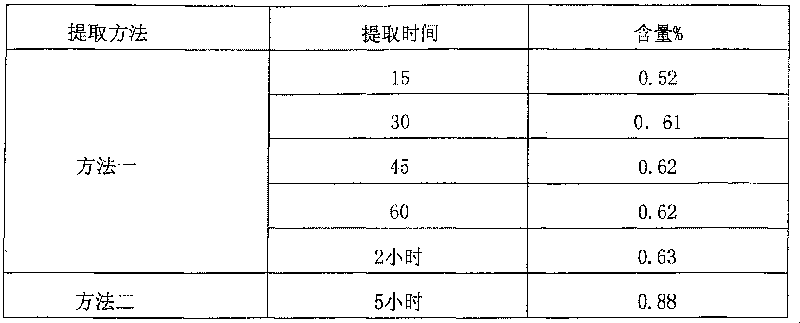
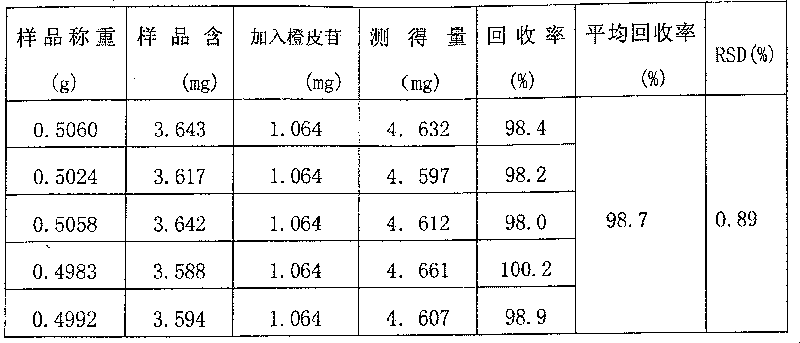
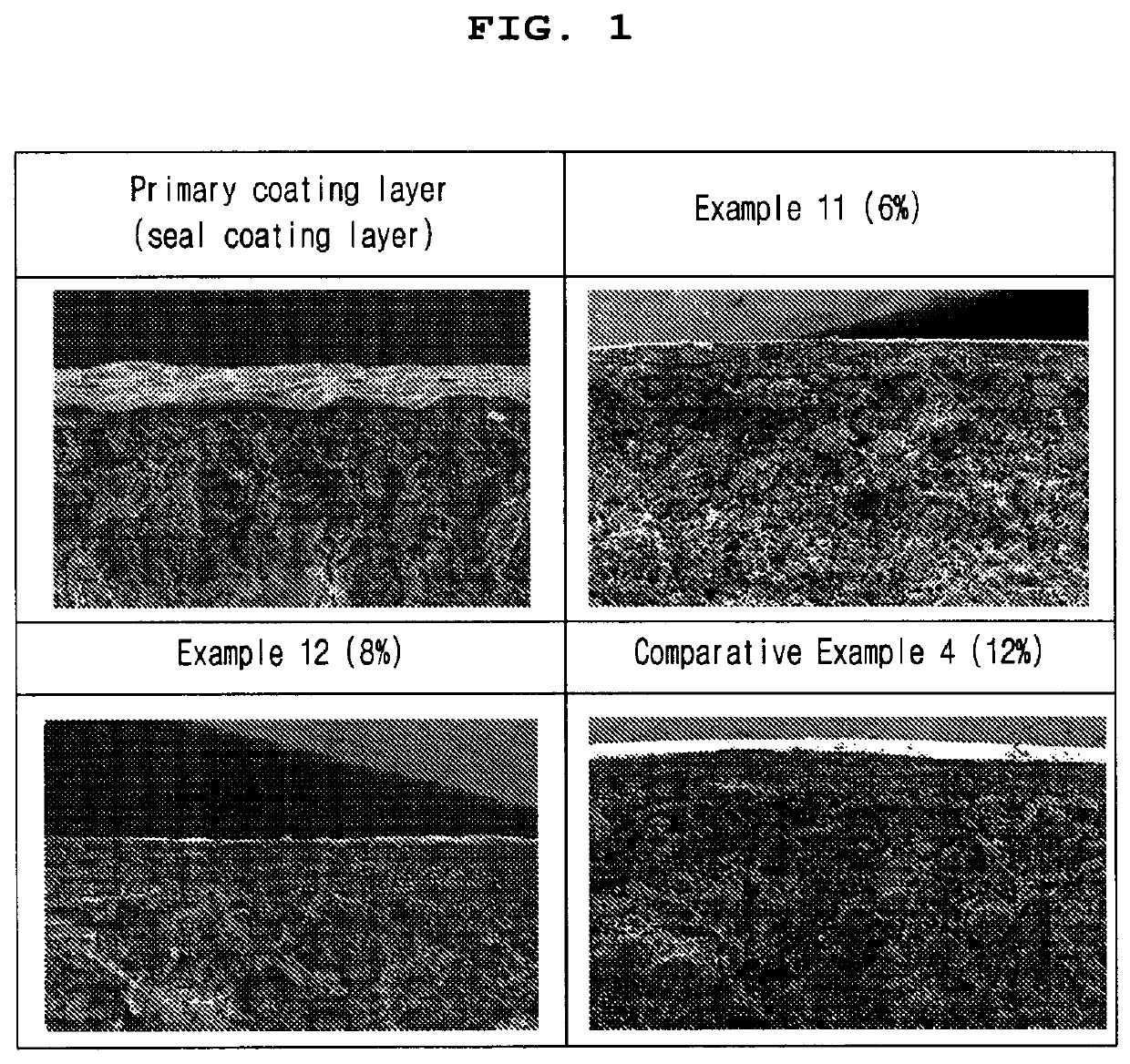
Patents
Literature
61 results about "Capsule Dosage Form" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
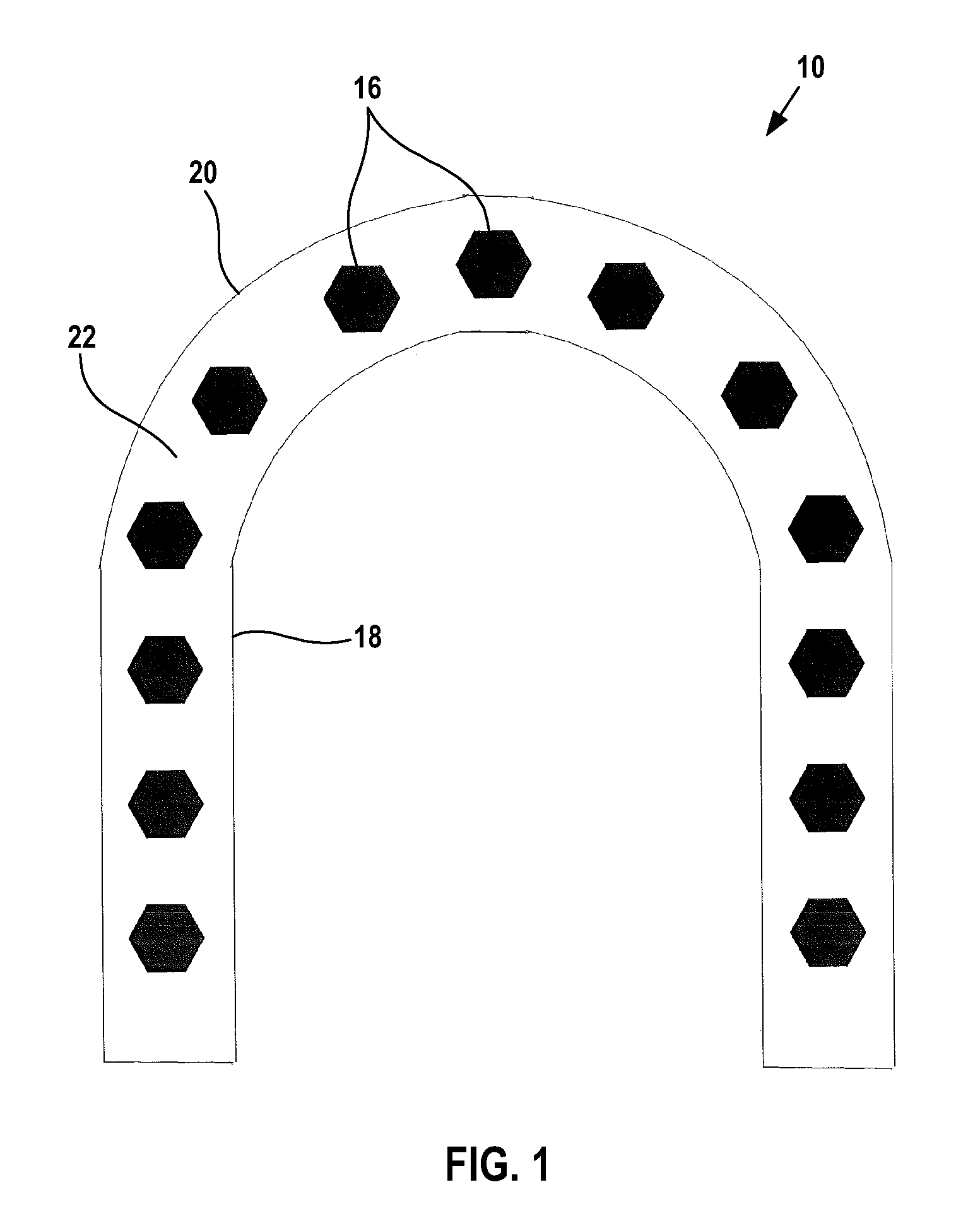
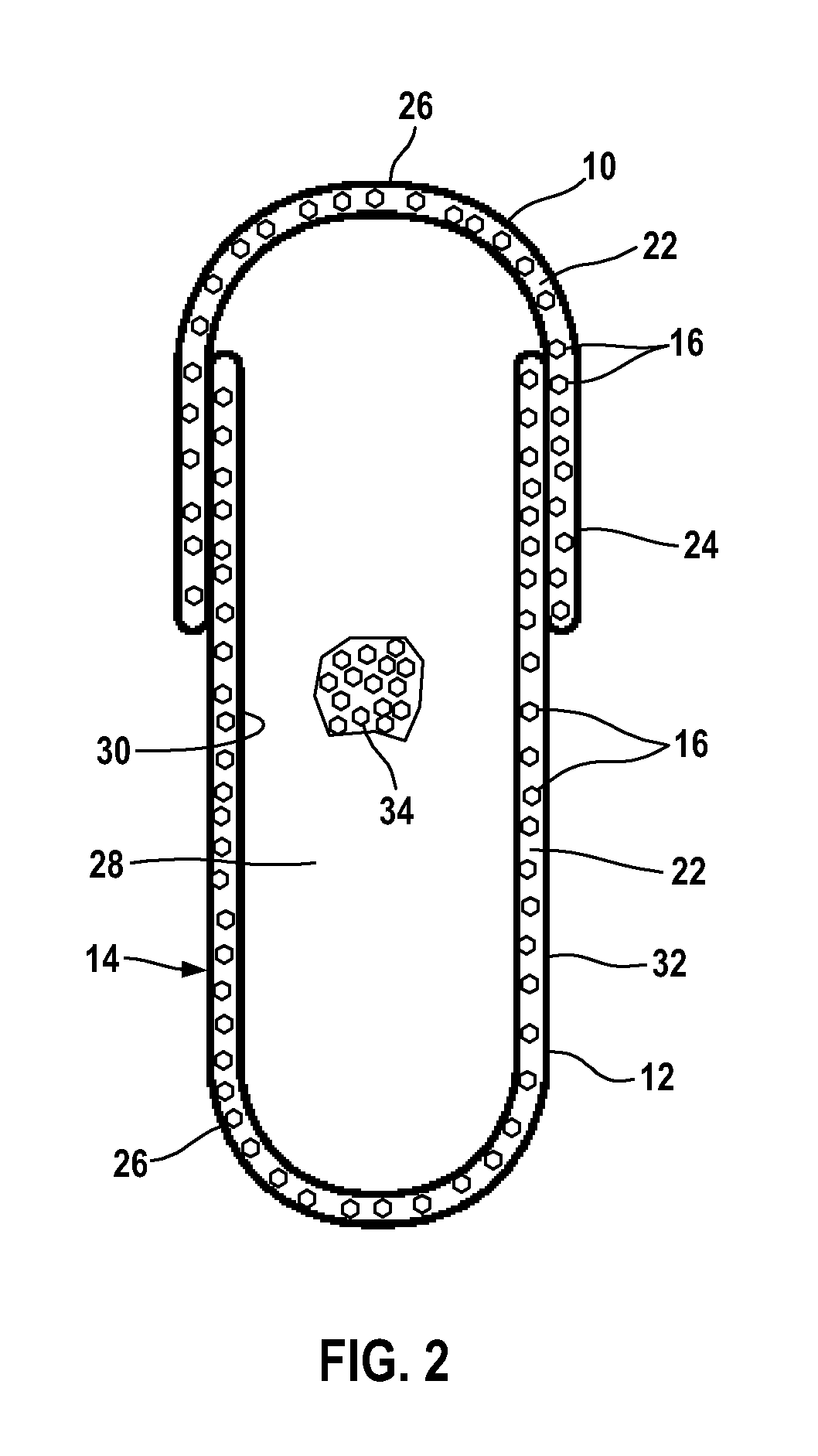
A solid pharmaceutical dosage form that contains medicinal agent within either a hard or soft soluble container or shell, usually used for the oral administration of medicine. The shells are made of a suitable form of gelatin or other substance. (NCI)
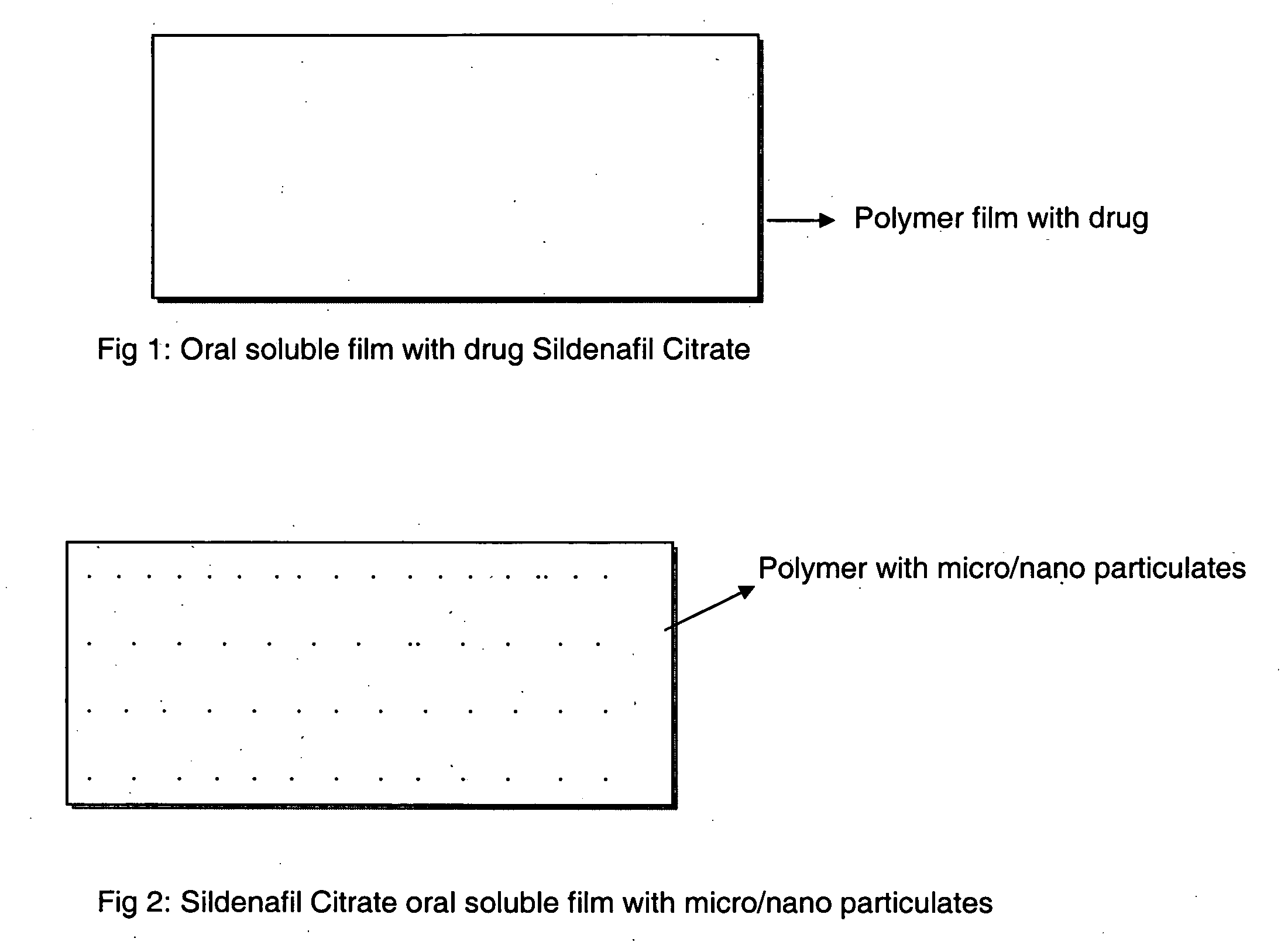
Oral fast dissolving films for erectile dysfunction bioactive agents
InactiveUS20090047330A1Improved ease of handlingIncrease usageBiocideAnimal repellantsVardenafilActive agent
A novel edible polymer based film dosage form manufactured using natural, synthetic, semisynthetic, pharmaceutically acceptable polymers addressing the issues of swallowing difficulties (Dysphagia and Dynaphagia), of tablet or capsule dosage forms and handling and storage difficulties associated with liquid dosage forms, that also includes materials such as emulsifying agents, suspending agents, buffering agents, effervescence agents, colorants, flavorants, sweeteners and specified amounts of bioactive agents, for erectile dysfunction. A flexible film dosage form containing sildenafil citrate, tadalafil or Vardenafil is presented. The film system is enabled to be used in various applications such as oral, mucosal and external environments.
Owner:BANGALORE RAMESH
Physically/molecularly distributed and/or chemically bound medicaments in empty, hard capsule shells
The present invention incorporates medicaments in the empty hard capsule shells (body and cap). The medicament is either physically / molecularly distributed and / or chemically bound to the polymer matrix of the capsule shell composition. Other medicaments in the form of drug-loaded matrices (powders, granules, beads, pellets, mini-tablets, and mini-capsules) can be filled in the drug-loaded empty, hard capsule shells. The same capsule dosage form contains medicaments in the core matrix and in the shell.
Owner:JOSHI HEMANT N +1
Coated pharmaceutical capsule dosage form
InactiveUS20120244216A1Improve oral bioavailabilityImprove solubilityAntibacterial agentsOrganic active ingredientsAdditive ingredientCapsule Dosage Form
Owner:SHAH MANISH S +1
Perforated water soluble polymer based edible films
InactiveUS20090047350A1High drug loadingImprove abilitiesPowder deliveryOrganic active ingredientsSolubilityActive agent
Owner:BANGALORE RAMESH
Coated pharmaceutical capsule dosage form
InactiveUS20100291201A1Improve oral bioavailabilityImprove solubilityBiocideNervous disorderAdditive ingredientCapsule Dosage Form
Owner:CEROVENE
Pharmaceutical compositions of mesalamine
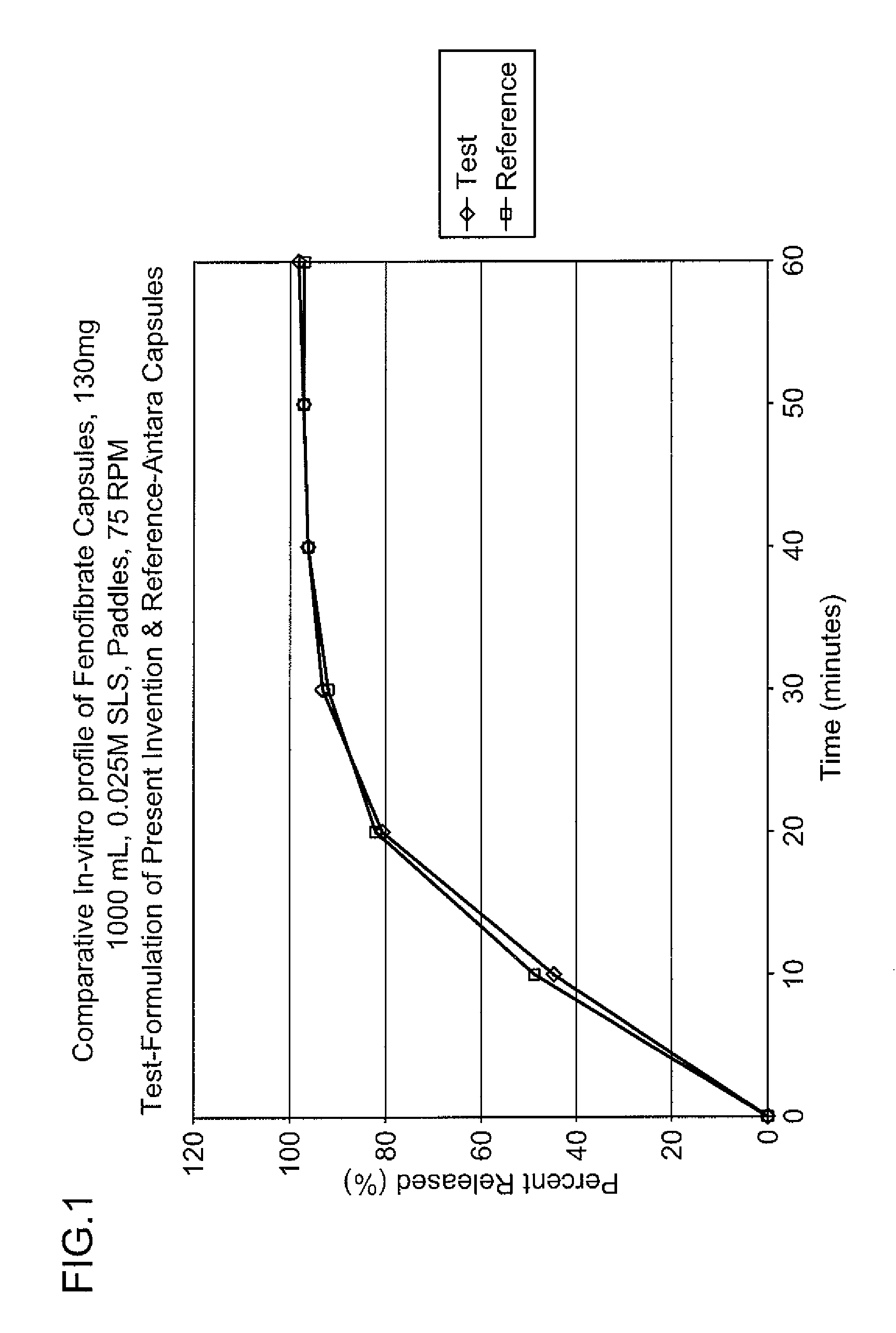
The present invention relates to pharmaceutical compositions of mesalamine. The composition of the invention is a capsule dosage form filled with a tablet. The invention also relates to process for preparing such compositions. The invention specifically relates to a composition comprising an effective amount of mesalamine having higher bulk density.
Owner:CADILA HEALTHCARE LTD
Hollow microcapsule, acidic or alkaline controlled-release microcapsule and preparation methods thereof
ActiveCN102744022AMaintain insecticidal activityBiocideAnimal repellantsInsecticidal crystal proteinsLayer by layer self assembly
The invention relates to a hollow microcapsule, an acidic or alkaline controlled-release microcapsule and preparation methods thereof, belonging to the field of biological agents. The preparation method of the hollow microcapsule comprises the following steps of: preparing a microcapsule through a layer-by-layer self-assembly method by taking calcium carbonate as a core and selecting polyelectrolyte cation and anion with opposite charges under a preparation condition; and then removing the core to obtain the hollow microcapsule. The wall of the hollow microcapsule is formed by alternating PAH / PSS (Polypropylene Amine hydrochloride / Poly Sodium Styrenesulfonate), and the hollow microcapsule can loaded with Cry protoxins of bacillus thuringiensis insecticidal crystal proteins under an acidic condition so as to obtain a microcapsule dosage form with release controlled under an alkaline condition. The microcapsule dosage form keeps the insecticidal activity of the cry protoxins, can assist the cry protoxins to resist the influence of certain environmental factors, also enables the insecticidal proteins to be specifically released in an alkaline environment of an insect midgut and keep stable in a general environment and plays an important role in the actual application of the Bt (Bacillus thuringiensis) insecticidal proteins on pest control.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Medicine for curing cerebrovascular diseases and its preparation method
InactiveCN1460483ALow toxicityPromote hematoma regressionOrganic active ingredientsSugar derivativesDiseaseVascular disease
The present invention relates to a medicine for curing cerebrovascular diseases and its preparation method. The effective component of said medicine is gardenia total iridoid glycoide extracted from Chinese medicinal material gardenia root, in which its main component is gardenaside. Said invention also provides dosage form of said medicine, specially provides its capsule preparation.
Owner:北京正青星光生物医学科技有限公司 +1
Physically/molecularly distributed and/or chemically bound medicaments in empty, hard capsule shells
The present invention incorporates medicaments in the empty hard capsule shells (body and cap). The medicament is either physically / molecularly distributed and / or chemically bound to the polymer matrix of the capsule shell composition. Other medicaments in the form of drug-loaded matrices (powders, granules, beads, pellets, mini-tablets, and mini-capsules) can be filled in the drug-loaded empty, hard capsule shells. The same capsule dosage form contains medicaments in the core matrix and in the shell.
Owner:JOSHI HEMANT N +1
Preparation method of Bawei Chenxiang capsule
The invention discloses a preparation method of a Bawei Chenxiang capsule, which comprises the following steps: (1) extracting volatile oil; (2) carrying out alcohol extraction; (3) preparing ox-hearts into freeze-dried powder; (4) carrying out a water extraction process; and (5) preparing a capsule dosage form. The invention has the advantages that the preparation method is scientific and reasonable, integrates modern separation and extraction technologies and extracts related effective components and positions of all medicines according to indication functions so that the efficacy of the medicines is fully applied and the Bawei Chenxiang capsule can achieve the efficacy of the powder of the original prescription with the half dosage of the powder of the original prescription. The capsule preparation avoids the bad odor of medicines and is conveniently taken and carried. After the capsule preparation is changed into capsules, the stability of the medicines is improved.
Owner:INNER MONGOLIA MEDICAL COLLEGE
A kind of medicine for treating infantile eczema and preparation method thereof
InactiveCN102266451AGood curative effectDefinite curative effectImmunological disordersDermatological disorderCodonopsisDouble-flowered
Owner:长治市郊区黄碾镇中心卫生院
Proprietary Chinese medicine preparations for curing depression after cerebral infarction
InactiveCN101249246AGood treatment complianceShort course of treatmentNervous disorderPill deliveryTherapeutic effectAdemetionine
The invention provides a Chinese patent medicine preparation for curing the depression after cerebral infarction. The invention comprises the drugs calculated according to the proportion by weight as follows: 10 to 20 parts of rhizome of Sichuan lovage, 6 to 10 parts of bupleurum root, 6 to 15 parts of polygala root, 10 to 20 parts of Chinese angelica root, 10 to 30 parts of root of herbaceous peony, 10 to 15 parts of curcuma root, 10 to 20 parts of safflower, 15 to 25 parts of arboruitae seed and 30 to 50 parts of parched wild jujube seed. The Chinese patent medicine preparation adopts the preparation method that the Chinese drug materials are ground into fine powder of more than 100 meshes according to the formulation, and are uniformly mixed, the ozone sterilization processing is performed for 20 minutes, the materials are mixed with honey and stirred uniformly, and then is processed into pill dosage form or capsule dosage form. The preparation is a pure herbal preparation, and has the advantages that the curative effect is remarkable, the production method is simple, the cost is low, the toxic and side effects are less, the preparation is easy to be accepted by the patient, the drugs are mutually combined and has the functions of dispersing and rectifying the depressed liver-energy, stimulating circulation to end stasis, eliminating sputum, inducing resuscitation, waking up the patient from inconsciousness and allaying restlessness, the preparation can be used for curing the depression after cerebral infarction with remarkable curative effect and no obvious toxic and side effects.
Owner:盛文化
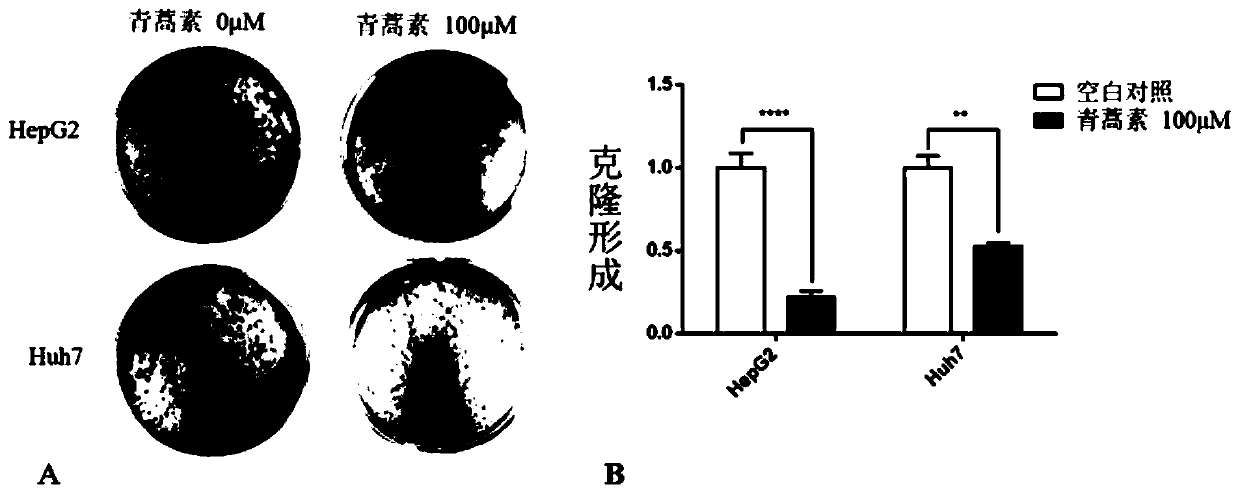
Application of artemisinin in preparation of drugs for resisting human liver cancer HepG2 and Huh7 cells
InactiveCN110151758AProliferation inhibitoryConvenient timeOrganic active ingredientsDispersion deliveryDrug capsuleHepg2 cells
The invention discloses application of artemisinin in preparation of drugs for resisting human liver cancer HepG2 and Huh7 cells. The purpose is achieved by inhibiting proliferation of the human livercancer HepG2 and Huh7 cells, and / or adjusting the expression profile of miRNA in the human liver cancer HepG2 and Huh7 cells. The concentration of the artemisinin is lower than or equal to 100 micromoles; the drugs are in an electuary dosage form or a capsule dosage form. The invention further discloses a drug electuary which contains the artemisinin and resists the human liver cancer HepG2 and Huh7 cells, a drug capsule which contains the artemisinin and resists the human liver cancer HepG2 and Huh7 cells and preparation method of the drug capsule. Under the effect of the artemisinin drugs,the proliferation of the human liver cancer HepG2 and Huh7 cells is inhibited, changes of the expression profile of the miRNA in the human liver cancer HepG2 and Huh7 cells are adjusted and controlled, the miRNA potentially adjusting and controlling the liver cancer is discovered, and the foundation is laid for strategic development of the artemisinin in the aspect of liver cancer treatment.
Owner:SOUTHERN MEDICAL UNIVERSITY
Traditional Chinese medicine preparation for treating megrim
InactiveCN106334148AEffective fastImprove subjective symptomsSenses disorderDigestive systemSide effectTherapeutic effect
A traditional Chinese medicine preparation for treating megrim comprises, by weight parts, 15-30 parts of poria cocos, 10-15 parts of rhizoma alismatis, 8-12 parts of dried orange peel, 10-15 parts of bighead atractylodes rhizome, 6-10 parts of ginseng, 10-15 parts of prepared pinellia tuber, 6-10 parts of cassia twig, 10-15 parts of rhizoma atractylodis, 6-10 parts of round cardamom, 10-15 parts of rhizoma gastrodiae, 10-12 parts of prepared licorice, 5-9 parts of safflower, 3-8 parts of Japanese eupatorium, 5-10 parts of uncaria and 8-13 parts of fructus tribuli. The raw materials are jointly smashed and processed into fine powder, ozone sterilization is performed for 20 min, unifom mixing is performed, and a capsule dosage form is manufactured. The traditional Chinese medicine preparation brings conventional advantages of traditional Chinese medicine into play, is obvious in treatment effect, a production method is simple and low in cost, and the traditional Chinese medicine preparation is easily accepted by patients. Through clinical experiments for many years, it is proved that the traditional Chinese medicine preparation becomes effectively quickly, subjective symptoms and signs are obviously improved, the traditional Chinese medicine preparation has no side effect, and the megrim does not recur easily.
Owner:李禾禾
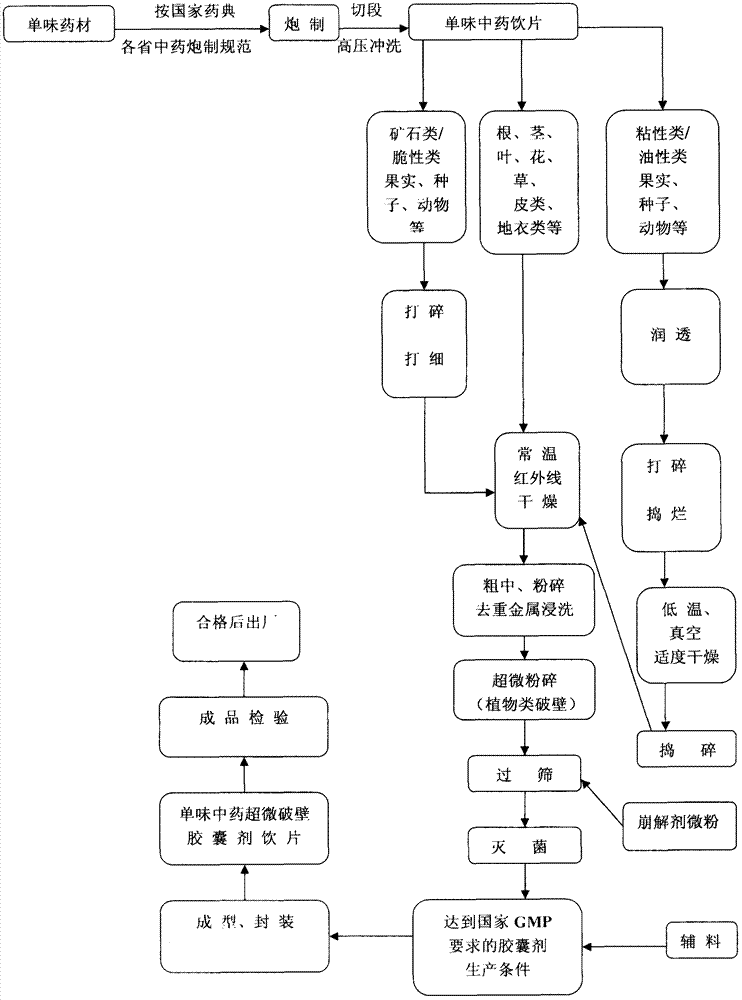
Processing method of ultra-micro wall-breaking capsule-preparation decoction pieces by traditional Chinese medicines
InactiveCN102772439AHigh drug loadingUniform and stable drug releaseCapsule deliveryAgainst vector-borne diseasesPesticide residueTraditional medicine
The invention discloses a processing method of ultra-micro wall-breaking capsule-preparation decoction pieces by traditional Chinese medicines, which mainly comprises the following steps that: pesticide residues are washed by using high pressure after single traditional Chinese medicinal materials are processed and cut in a standardization way, the traditional Chinese medicinal materials are processed into coarse powder after being dried, heavy metal is removed by dipping, then the coarse powder is processed into ultra-micro wall-breaking powder by ultra-micro pulverization, afterwards the powder is sterilized after being combined with the micro-powder of medicinal auxiliary materials with a certain proportion after quality control reaches a standard, and finally, the step of preparing the capsule-preparation decoction pieces is finished. According to the processing method of the ultra-micro wall-breaking capsule-preparation decoction pieces by the traditional Chinese medicines, the decoction pieces of single traditional Chinese medicines are prepared into a novel preparation of the capsule-preparation decoction pieces, which can be directly swallowed without infusion. According to the preparation, the advantages of ultra-micro powder and a capsule preparation are fused, the time of acting can also be controlled, the use quantity of the auxiliary materials is little, the medicine loading quantity is high, and a novel method for modernizing traditional Chinese medicines is initiated.
Owner:周明千
Pidotimod capsule preparation and preparation thereof
InactiveCN101234096AFast absorptionImprove bioavailabilityDipeptide ingredientsPharmaceutical non-active ingredientsThiazoleDecomposition
Owner:沈阳双鼎制药有限公司
Method for detecting piperine in Chinese patent medicine for weight loss and health food
InactiveCN110082450AExclusive preliminary screeningSensitive primary screeningComponent separationUltravioletColumn temperature
The invention provides a method for detecting piperine in Chinese patent medicine for weight loss and health food. An ultra-high performance liquid chromatography-ultraviolet spectrum-time of flight mass spectrometry is adopted to carry out qualitative screening and content determination. Chromatographic conditions comprise that a chromatographic column is a C18 chromatographic column; a detectoris a diode array detector; a detection wavelength is 340nm; a scanning wavelength range is 190-400nm; a mobile phase includes A-0.1% formic acid solution and B-0.1% formic acid-acetonitrile; a flow rate is 0.3-0.4mL / min; a column temperature is 25-40 DEG C; a sample injection amount is 0.1-2muL; and an elution gradient program includes 10% B for 0-2min; 90% B for 7-9min;and 10% B for 9.1-10min. Byusing the method, exclusive, sensitive and rapid primary screening can be performed, and the piperine added in the Chinese patent medicine for weight loss and the health food is confirmed. The methodis mainly suitable for analysis of the piperine in tablets and capsule dosage forms.
Owner:苏州市药品检验检测研究中心
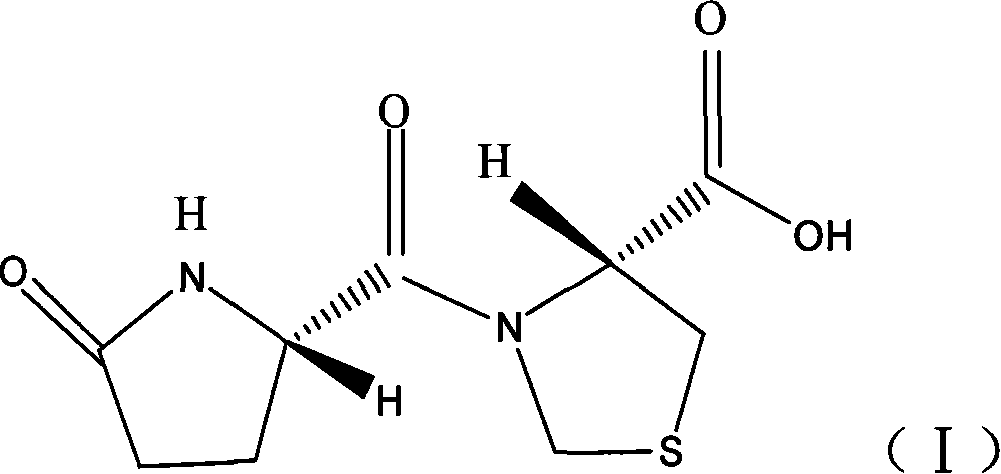
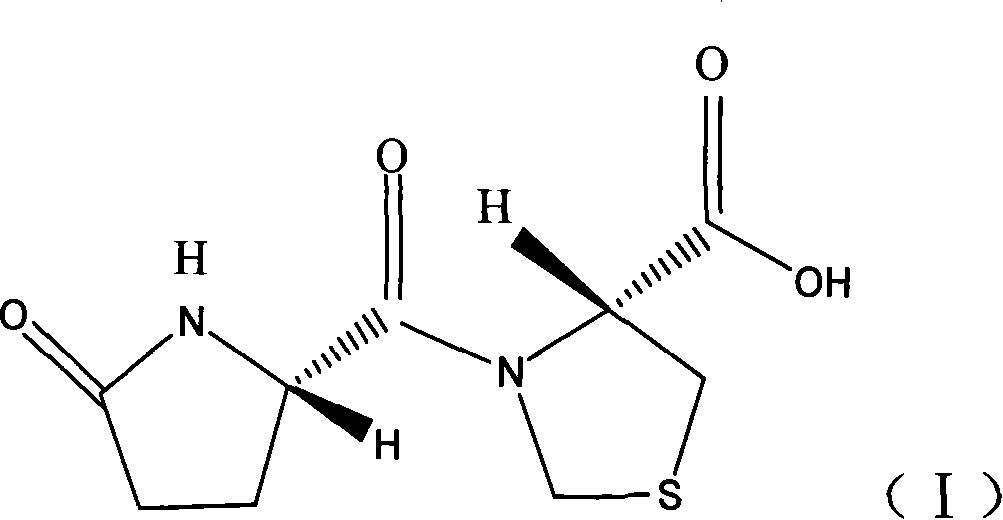
Capsule of corydalis yanhusuo extract for treating cardio vascular disease, and its preparing and effective ingredient content measuring method
InactiveCN1813877AMask bad smellPrevent and slow down the rate of degradationComponent separationCapsule deliverySustained Release CapsuleAdditive ingredient
The present invention provides a kedaling capsule preparation for effectively curing angiocardiopathy and cerebrovascular disease. Said capsule preparation is made up by using corydaline (or chlorinated dehydrocorydaline) as main component and adding conventional additive through a conventional capsule preparation process.
Owner:SICHUAN GUOKANG PHARMA
Preparation method of nitrochloroform capsule
ActiveCN108353917ASolve the problem of large loss and harsh application environmentSimple production processBiocideFungicidesSuccinic acidChemistry
The invention discloses a preparation method of a nitrochloroform capsule. The preparation method comprises the following steps: (1) taking sodium alginate solid or the sodium alginate solid and an auxiliary material to obtain a wall material water solution with the mass concentration of 0.5 to 3.5 percent, and standing for 24 hours for later use; (2) putting a core material nitrochloroform into the wall material water solution, adding a proper amount of emulgator tween 80 or tween 60, tween 20 and octenyl succinic acid modified starch, and performing high-speed emulsion dispersion for 30 to 60min to obtain emulsified liquid; (3) preparing the obtained emulsified liquid into the nitrochloroform capsule by adopting a piercing-solidifying method. The product is used for solving the existingproblem that in a process of applying nitrochloroform, the drug loss amount is great and the application environment is bad; in a process of applying the nitrochloroform capsule, peculiar smell is avoided, and a person applying the drug does not need special safeguard procedures, the production process of the capsule is simple and convenient, the production is low, the drug volatilization amount can be reduced by a capsule coating technology, the drug application cost is reduced, and remarkable environmental benefit and economic benefit are achieved.
Owner:DALIAN POLYTECHNIC UNIVERSITY
Traditional Chinese medicine preparation for curing cancer
InactiveCN101579497AAchieve balanceAchieve normal operationCapsule deliveryAntineoplastic agentsEsophagus CancersAtractylis ovata
The invention discloses a traditional Chinese medicine preparation for curing cancer, namely a detoxicating pill and a preparation method thereof. The traditional Chinese medicine preparation mainly comprises the following medicines according to parts by weight: 30-300 of paris polyphylla, 20-200 of pseudo-ginseng, 10-100 of trigone, 10-100 of rhizoma zedoariae, 20-200 of radix psammosilene, 20-200 of atractylis ovata, 10-100 of tuckahoe, 10-100 of rosin and 20-200 of safflower root. The invention provides a preferred preparation method of the traditional Chinese medicine preparation according to the requirements of prescription medicine properties and capsule dosage forms. The traditional Chinese medicine preparation and the preparation method thereof are a summary of clinical experience for many years by an inventor and have the special effects of curing gastric cancer, esophagus cancer, colon cancer, rectum cancer, and the like.
Owner:刘人源
Traditional Chinese medicine preparation for curing femoral head necrosis and vasculitis
The invention discloses a traditional Chinese medicine preparation for curing femoral head necrosis and vasculitis, namely a bone magic pill and a preparation method thereof. The traditional Chinese medicine preparation is formed mainly by mixing the following medicines: deer bone, achyranthes, pseudo-ginseng, rosin, honeysuckle, radix rehmanniae, licorice and dandelion. The invention provides a preferably preparation method of the traditional Chinese medicine preparation according to the requirements of prescription medicine properties and capsule dosage forms. The traditional Chinese medicine preparation and the preparation method thereof are a summary of clinical experience for many years by an inventor and have the special effects of curing the femoral head necrosis and the vasculitis.
Owner:刘人源
Medicament for treating dementia and preparation method thereof
InactiveCN101590163ARelieve symptomsImprove brain functionPowder deliveryNervous disorderSide effectBile fluid
The invention relates to a medicament for treating dementia and a preparation method thereof. The medicament is prepared from radix-polygoni multiflori, epimedium herbs, yams, tuckahoe, prepared rhizome of rehmannia, root barks of peony trees, alisma orientale, dogwood fruit, acorus gramineus and arisaema cum biles and usually prepared into a capsule dosage form and has good curative effect on the dementia. Through clinical treatment and observation, for the treatment to the dementia and compared with medicaments of the same class, the medicament not only has obviously improved curative effect, but also has no toxic side effect, and under the condition of lack of effective treatment medicaments to the dementia clinically at present, the medicament has significant medical value.
Owner:丁大愚
Preparation method of periplaneta americana active peptide
InactiveCN106581635AAnti-tumorCardiac BoostAntibacterial agentsPowder deliveryTissue repairFreeze-drying
The invention relates to the technical field of traditional Chinese medicine and biology, in particular to a method for preparation of periplaneta americana active peptide from periplaneta Americana. The method includes: selecting dried and fresh imagoes of periplaneta americana as the raw materials, conducting crushing homogenizing, enzymolysis, fishy smell removal, enzyme inactivation, centrifugation, sterilization and ultrafiltration, then according to oral solution production practices, performing filling to obtain oral solution dosage forms; making dry powder according to spray drying or freeze drying production process practices, and performing filling to obtain capsule dosage form and bottled freeze-dried powder dosage form. The nutritional value, the absorption and utilization rate and food safety are greatly improved. Also the periplaneta americana active peptide has anti-tumor, immunity enhancing, tissue repair, antibacterial, antiviral, heart strengthening and pressure boosting, microcirculation improving and other pharmacological activities. The preparation method of the periplaneta americana active peptide has the characteristics of rigorous and reasonable process and strong operability, and is easy for realizing industrial production.
Owner:赵德润
Pharmaceutical compositions of rivaroxaban
There is provided a dosages form comprising rivaroxaban and one or more pharmaceutically acceptable excipients. The present invention also provides a stable capsule dosage form comprising rivaroxaban and one or more pharmaceutically acceptable excipients. The invention also relates to process of preparation of such compositions.
Owner:MANKIND PHARMA LTD
Capsule dosage form of metoprolol succinate
The present invention provides an extended-release capsule dosage form of metoprolol succinate in the form of coated discrete units and processes for their preparation.
Owner:SUN PHARMA INDS
Improved preparation of Juhong pill, its preparation method and quality inspection method
The invention relates to a reddish orange capsule prepared from the following steps: (1) proportioning 15 kinds of medicinal materials, disintegrating dried orange peel into mesh fines, sieving, extracting the available compositions with dissolvent, making thick grease from the extract, (2) charging dried orange peel into the thick grease, mixing homogenously, drying into dried grease, and disintegrating the dried grease into mesh fines, (3) charging auxiliary materials and mixing homogeneously to obtain particles, (4) making capsules from the particles.
Owner:SICHUAN MEDCO PHARML
A kind of medicine for treating mammary gland hyperplasia and preparation method thereof
InactiveCN102266530ADefinite curative effectNo side effectsUnknown materialsSexual disorderClematisCurative effect
The invention discloses a medicine for treating mammary gland hyperplasia. , Shanjia Tablets as raw materials, the medicine can be made into a variety of medicaments for clinical use, with capsules as the preferred dosage form, the medicine of the present invention has good curative effect on various hyperplasia of mammary glands, and the clinical application is safe and reliable.
Owner:长治市郊区黄碾镇中心卫生院
Medicament for treating dementia and preparation method thereof
InactiveCN101590163BEasy to takeComply with health law regulationsPowder deliveryNervous disorderDevergie's diseaseSide effect
The invention relates to a medicament for treating dementia and a preparation method thereof. The medicament is prepared from radix-polygoni multiflori, epimedium herbs, yams, tuckahoe, prepared rhizome of rehmannia, root barks of peony trees, alisma orientale, dogwood fruit, acorus gramineus and arisaema cum biles and usually prepared into a capsule dosage form and has good curative effect on the dementia. Through clinical treatment and observation, for the treatment to the dementia and compared with medicaments of the same class, the medicament not only has obviously improved curative effect, but also has no toxic side effect, and under the condition of lack of effective treatment medicaments to the dementia clinically at present, the medicament has significant medical value.
Owner:丁大愚
Capsule dosage form of metoprolol succinate
ActiveUS9700530B2Easy to manageOrganic active ingredientsDispersion deliverySustained Release Capsule Dosage FormExtended Release Capsule
Owner:SUN PHARMA INDS
Enteric tablet containing dimethyl fumarate
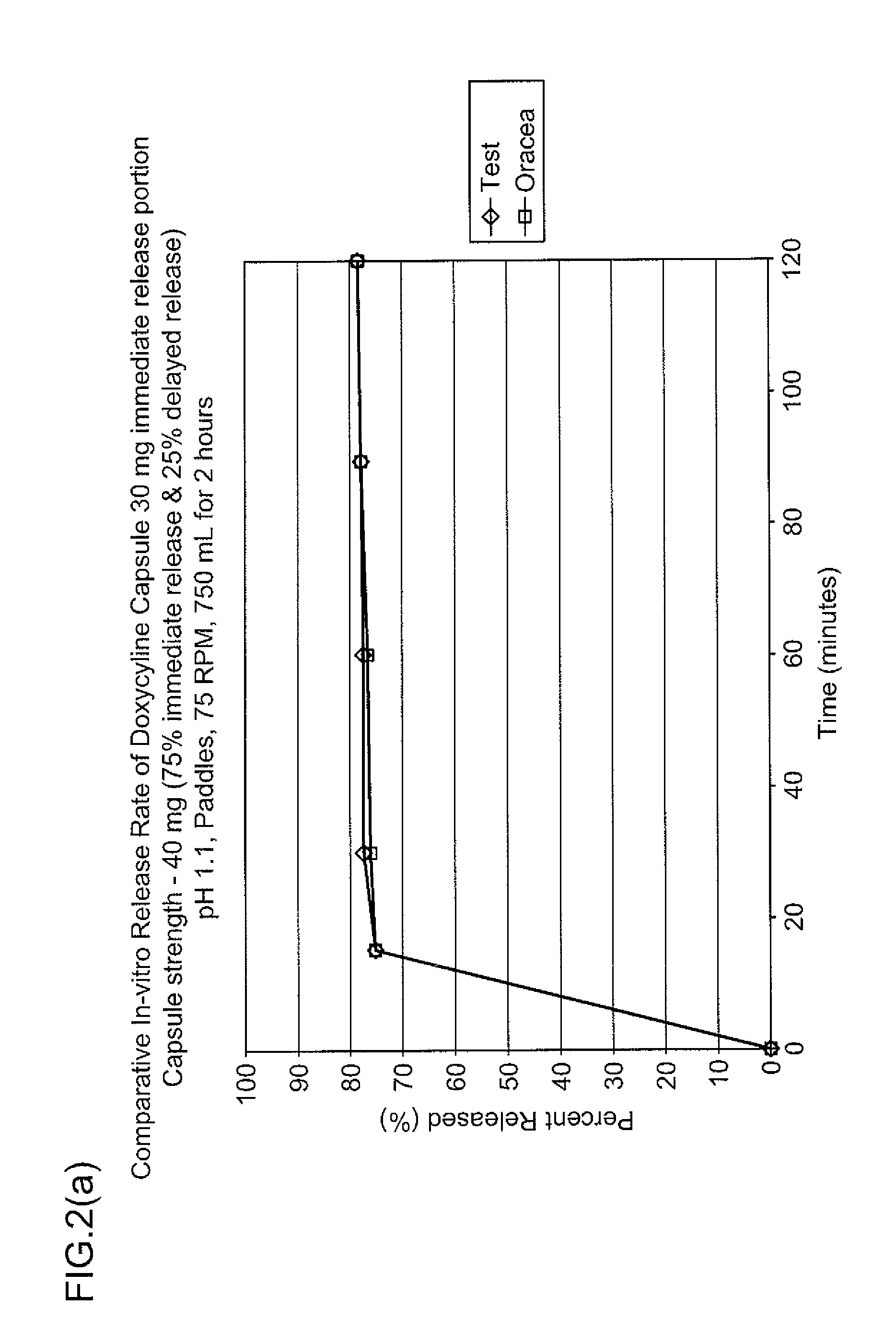
PendingUS20220087942A1Improve bioavailabilitySolve the real problemOrganic active ingredientsSenses disorderCaplet Dosage FormPharmaceutical medicine
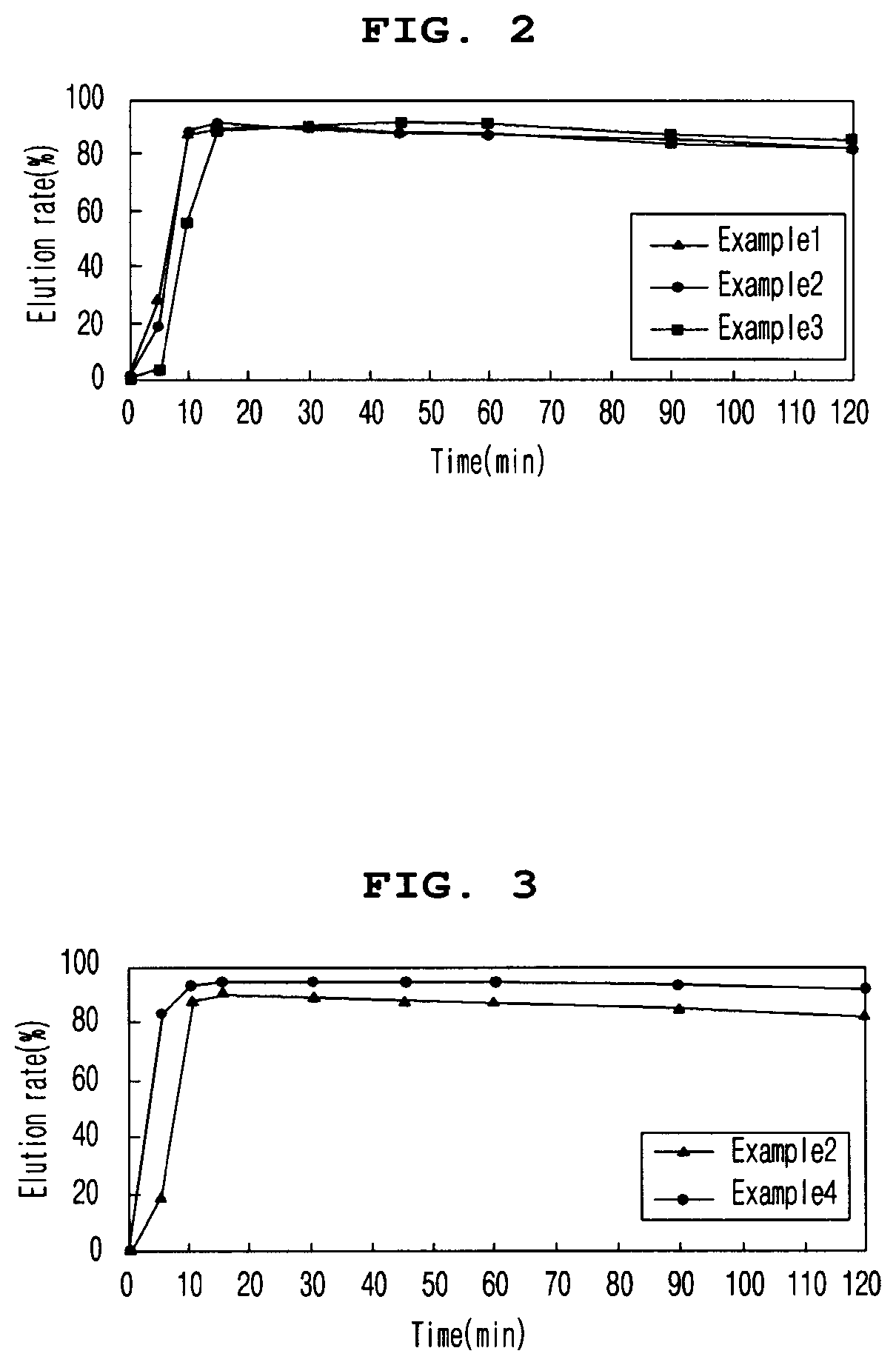
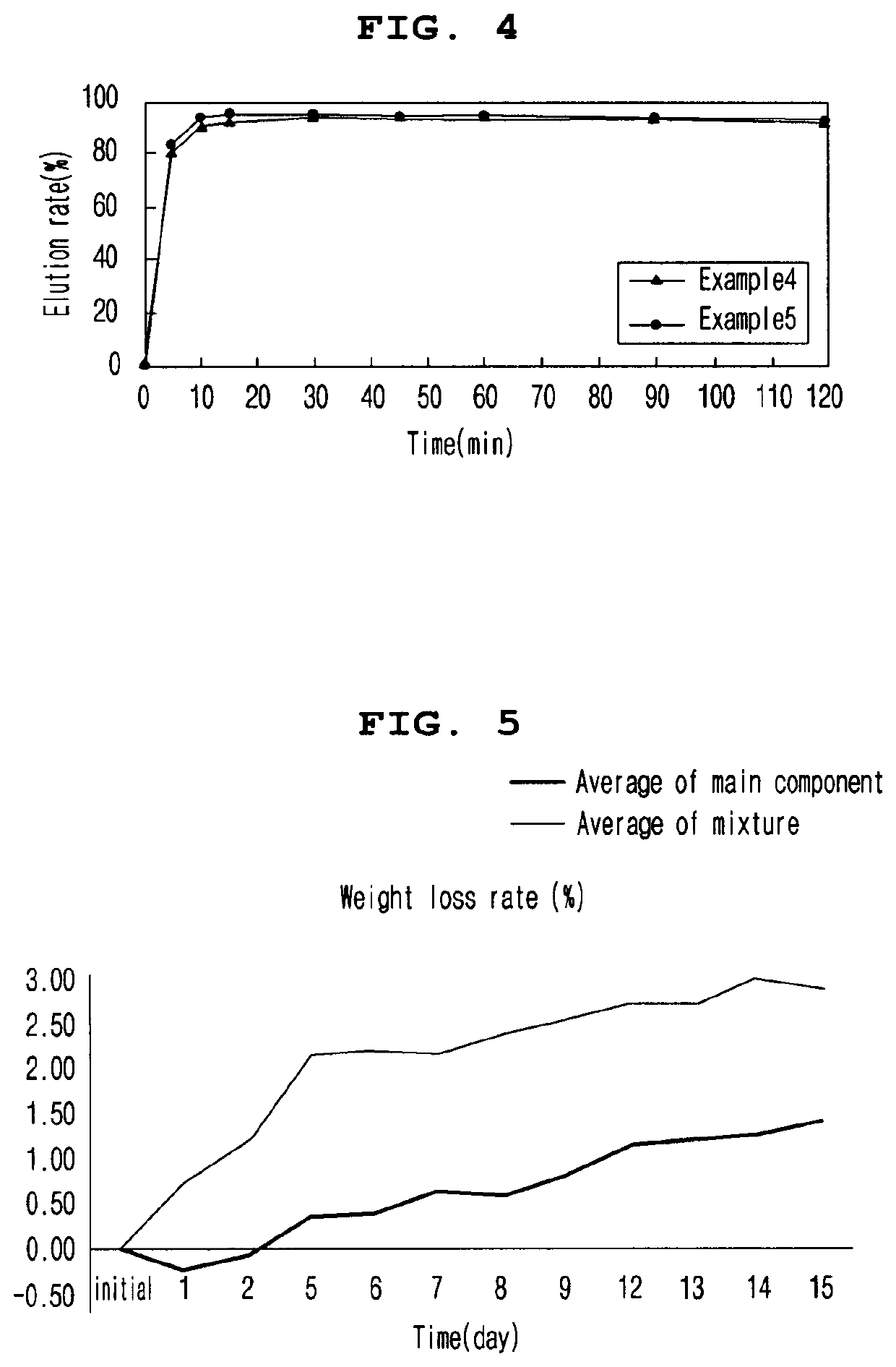
The present invention relates to an enteric coating tablet comprising: a core containing, as an active ingredient, dimethyl fumarate or a pharmaceutically acceptable salt thereof; and an enteric coating layer, and provides a tablet, which exhibits an effect equal to that of a capsule dosage form currently on the market, can be prepared through a simple preparation process, and is a dosage form having excellent storage stability and administration convenience, and thus can be applied to various patient groups.
Owner:CURACLE CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com