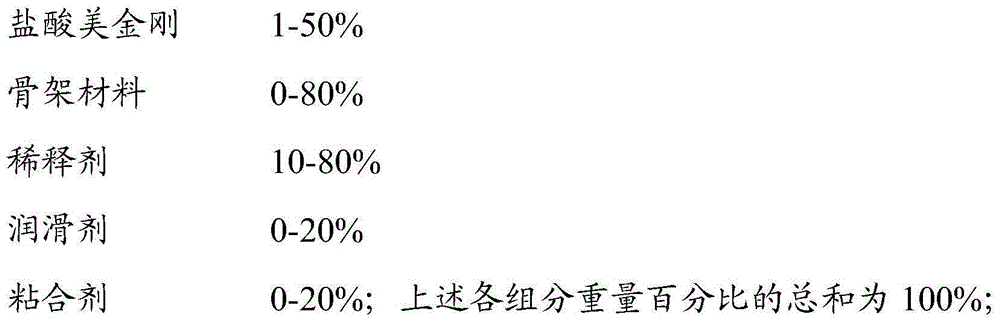Patents
Literature
38 results about "Mini tablets" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
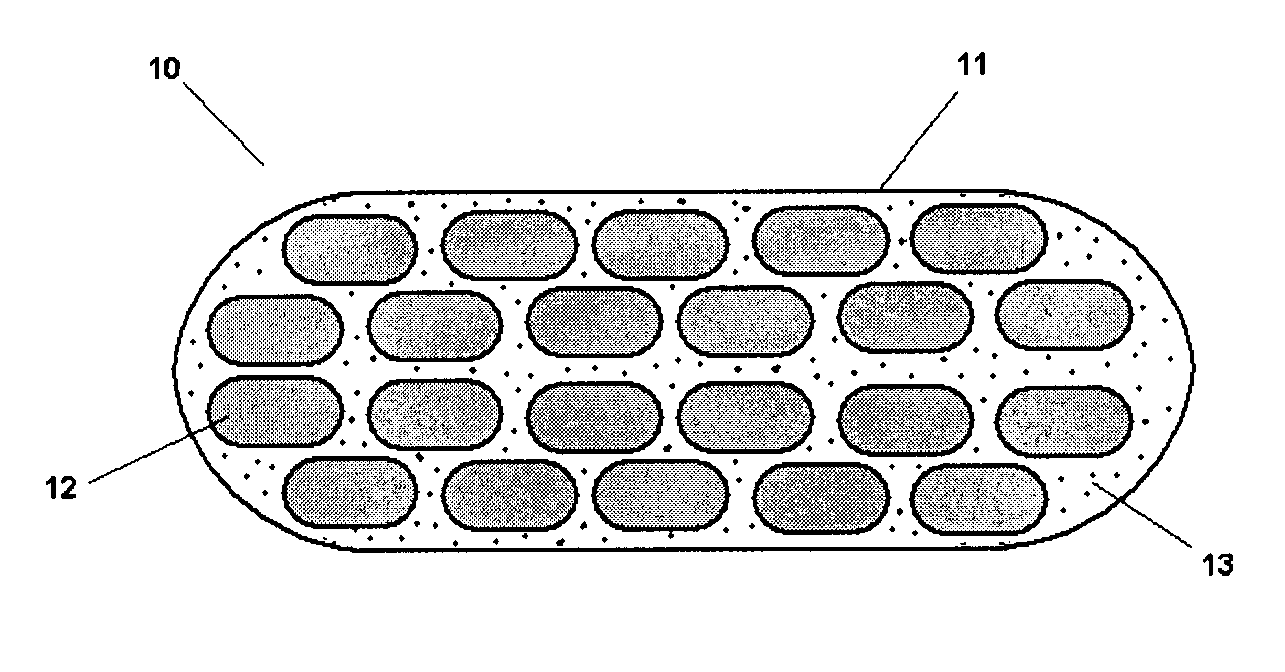
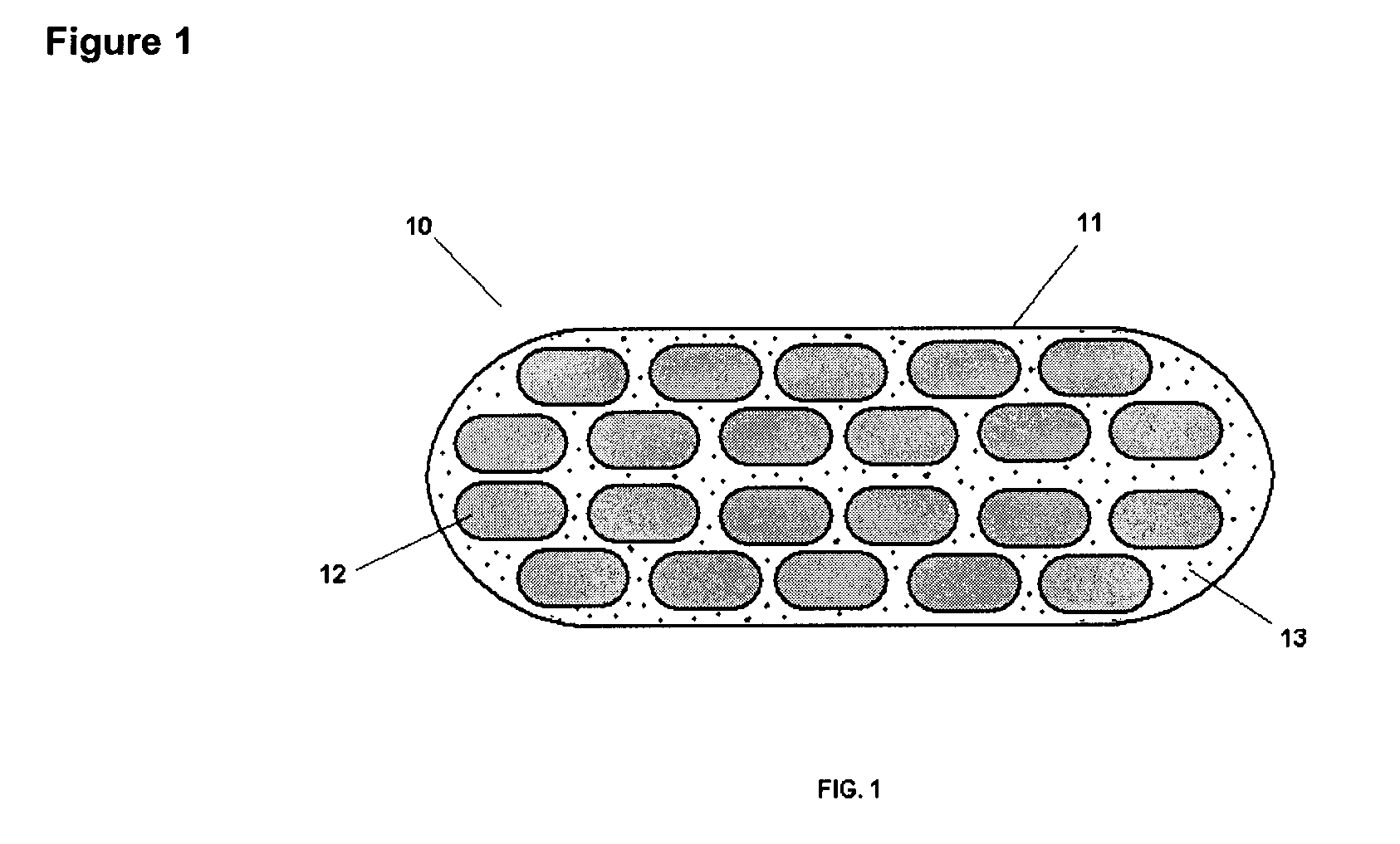
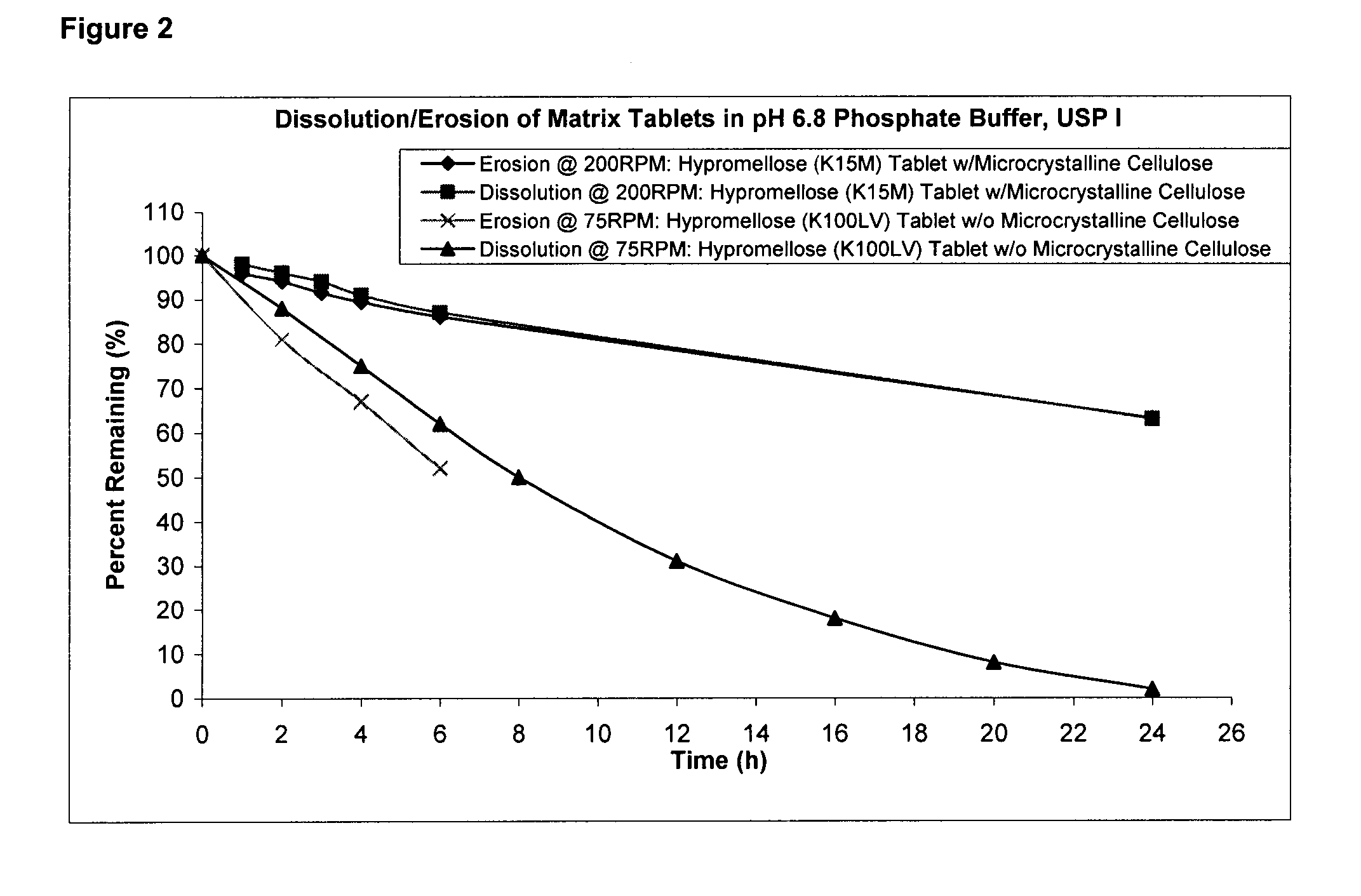
Pharmaceutical composition and administrations thereof
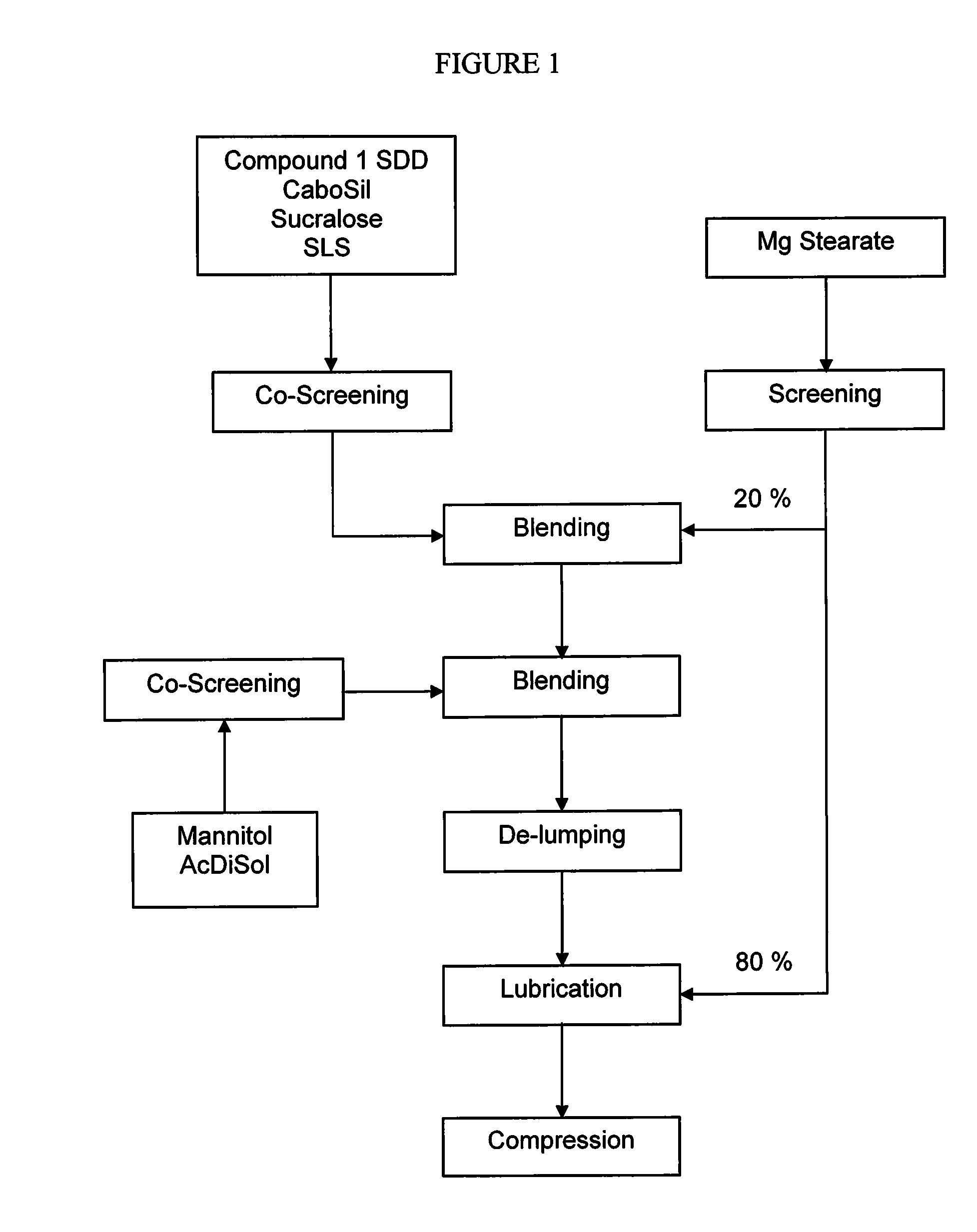
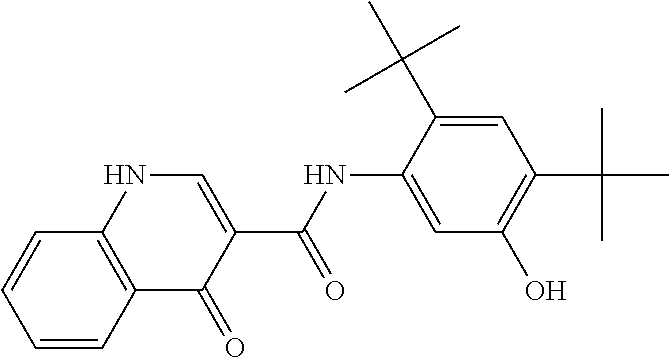
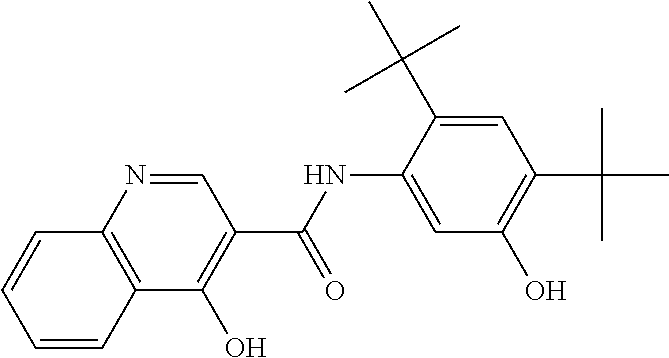
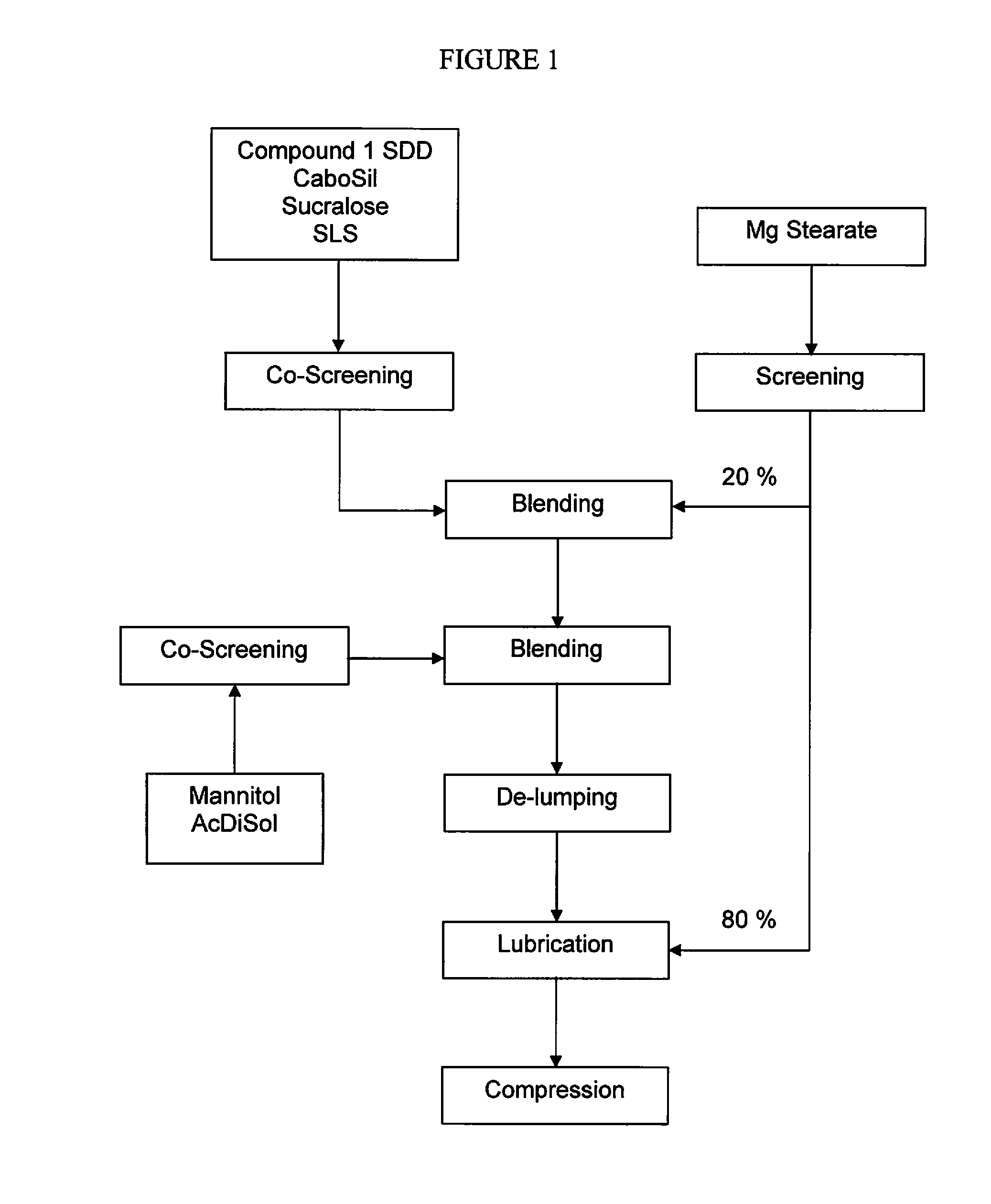
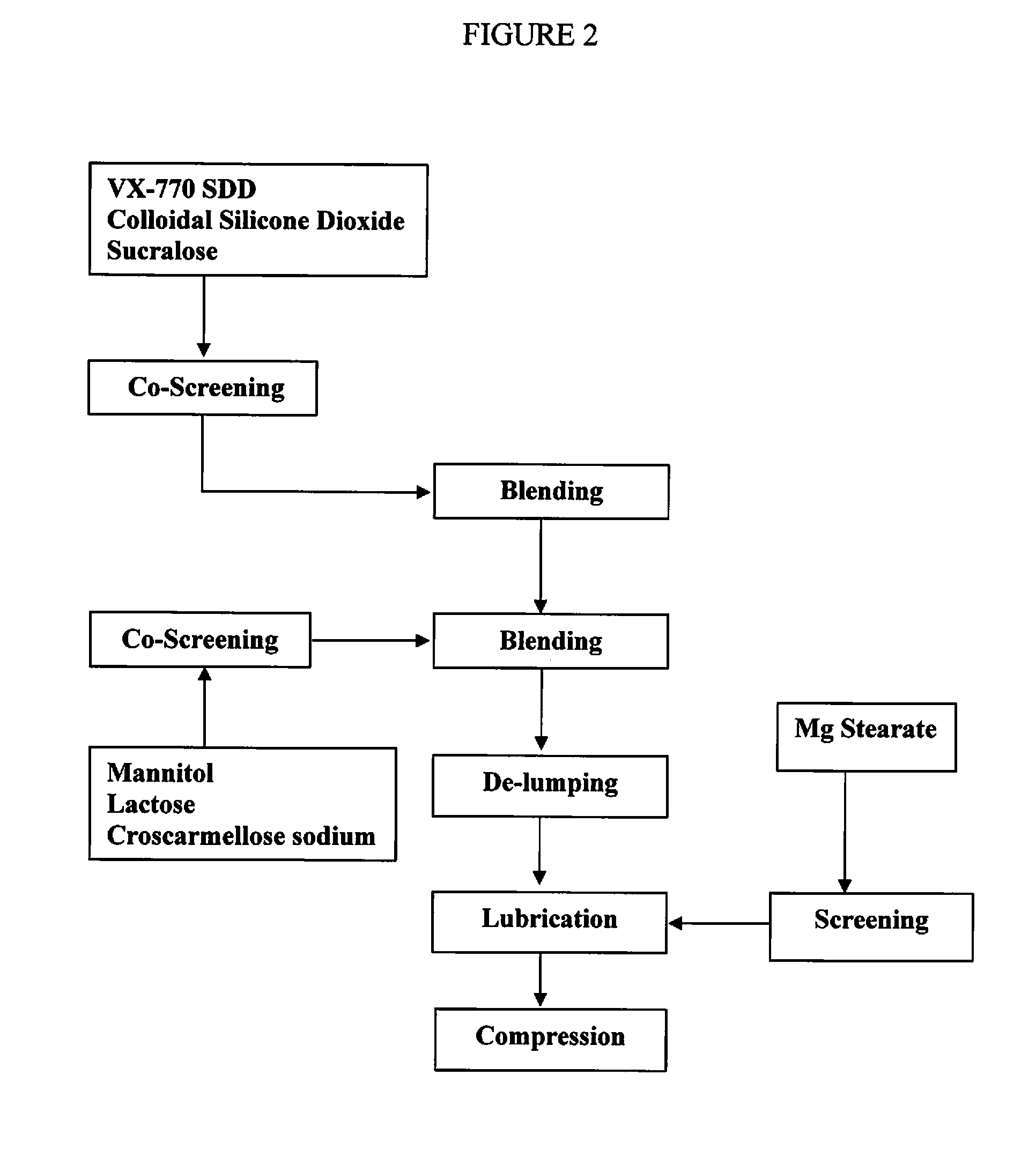
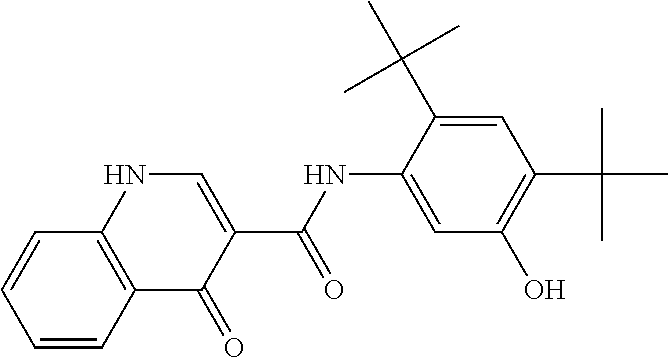
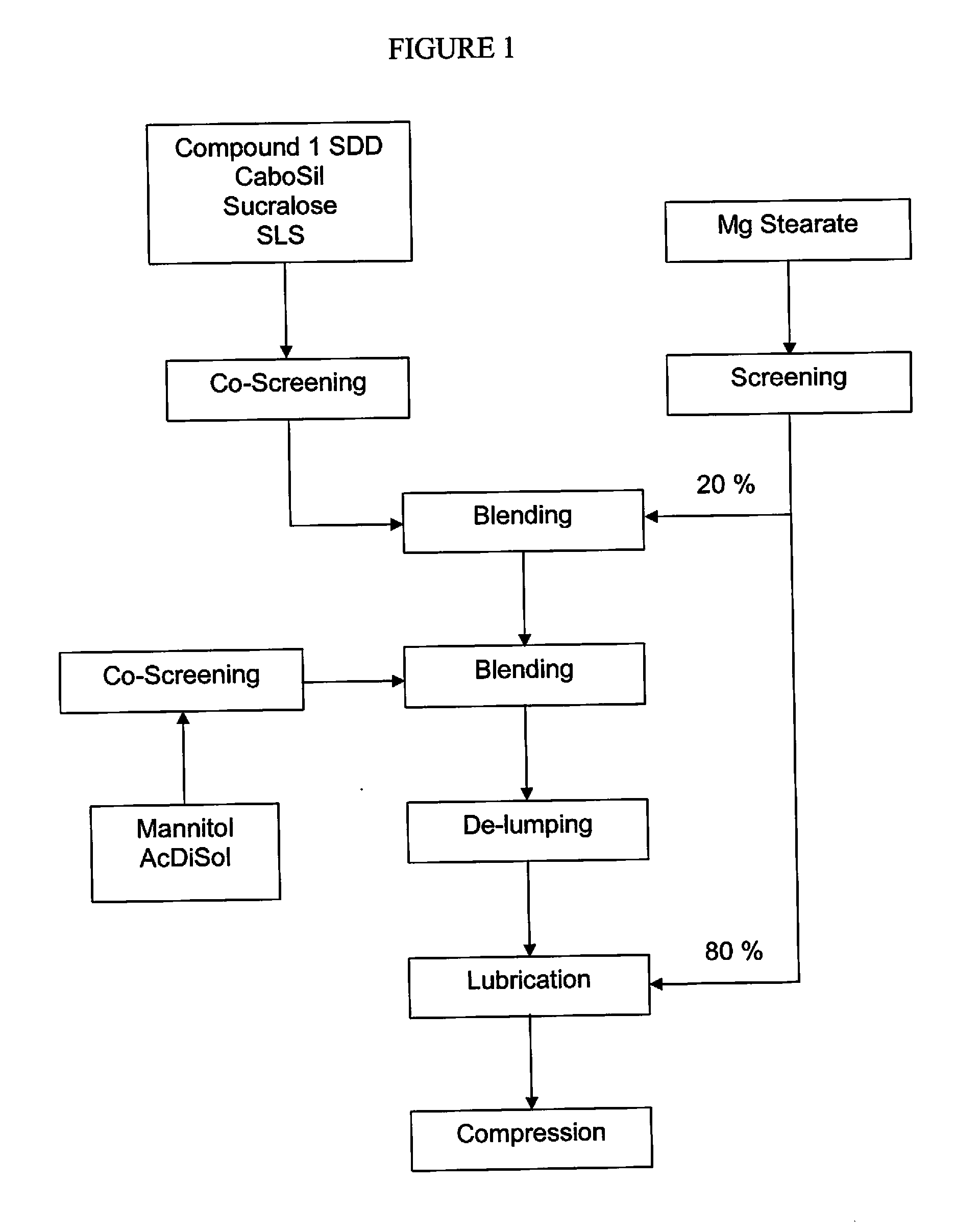
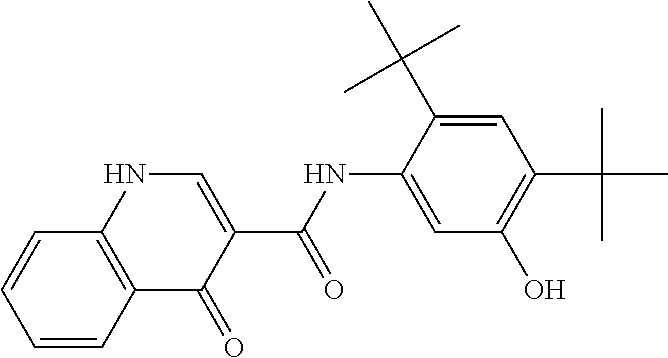
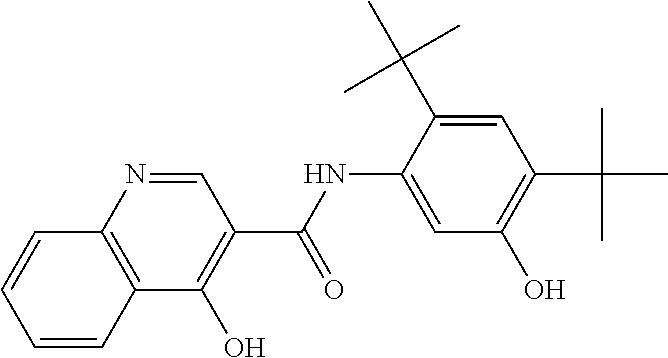
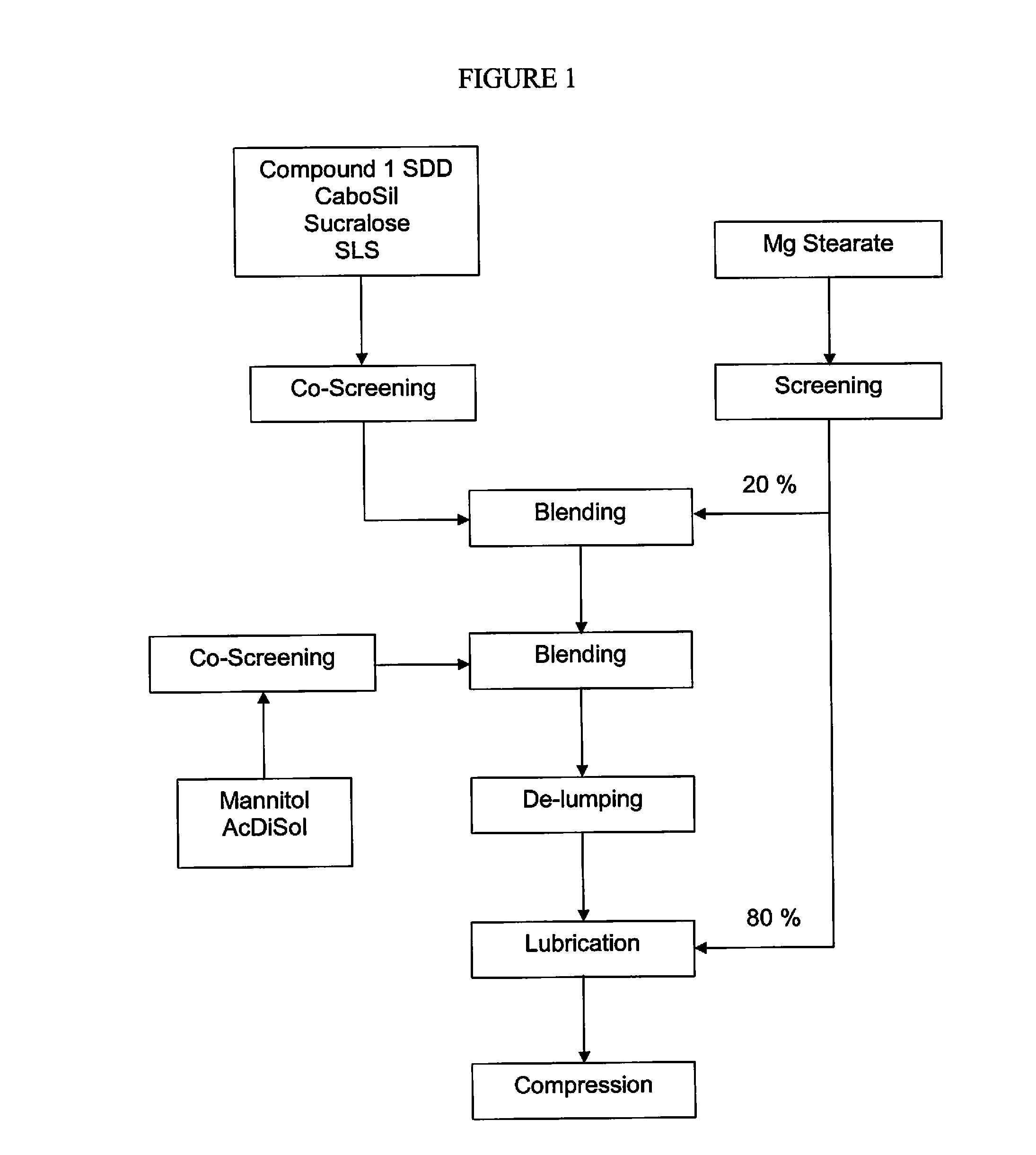
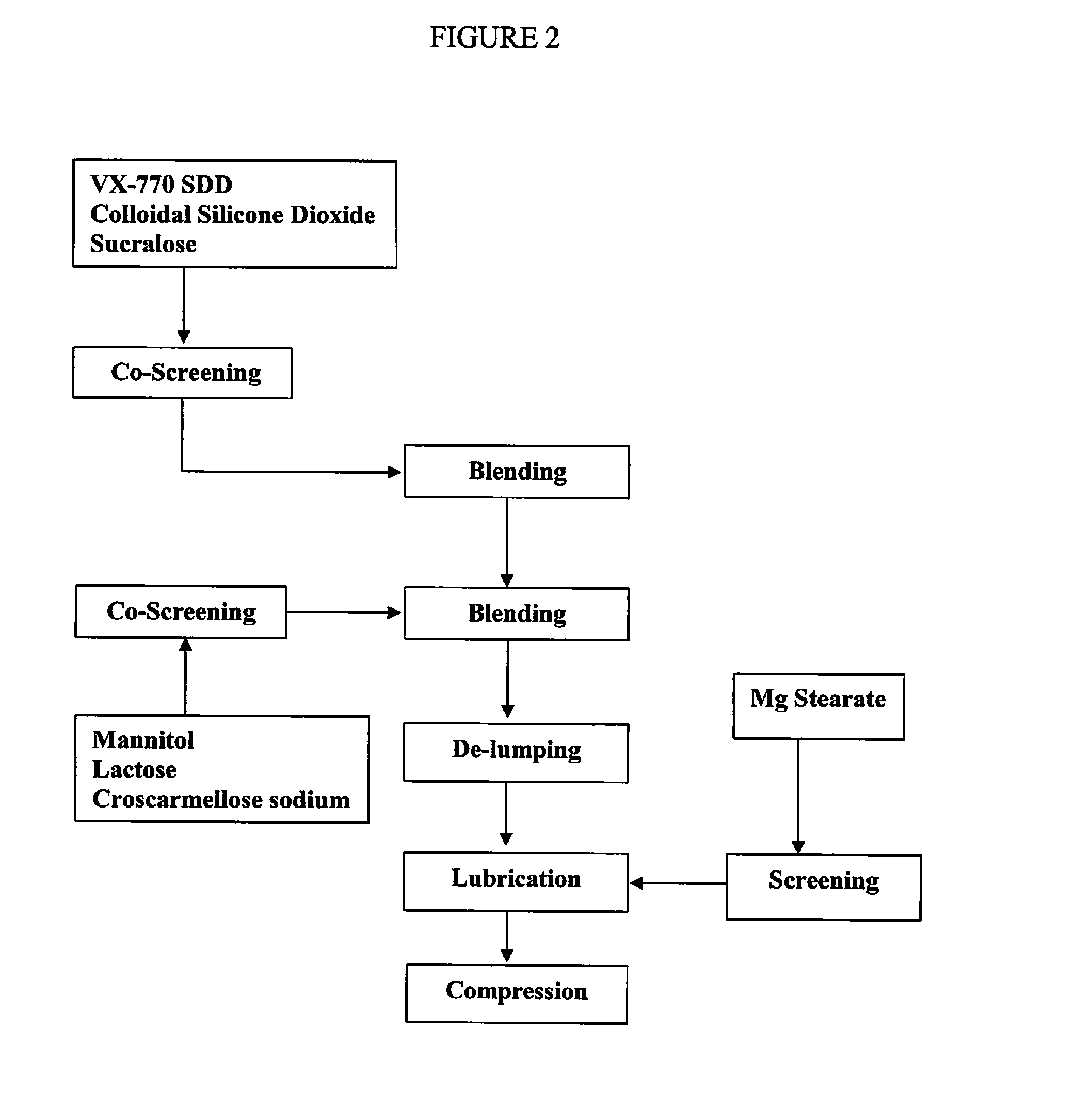
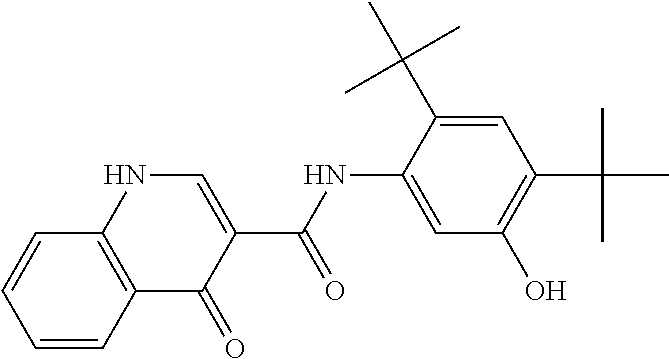
The present invention relates to pharmaceutical compositions containing a solid dispersion of N-[2,4-Bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide including formulations of the solid dispersions into powders, granules and mini-tablets, methods for manufacturing and processing the powders and mini-tablets and methods for treating cystic fibrosis employing the pharmaceutical composition.
Owner:VERTEX PHARMA INC
Pharmaceutical composition and administrations thereof
The present invention relates to pharmaceutical compositions containing a solid dispersion of N—[2,4-Bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide including formulations of the solid dispersions into powders, granules and mini-tablets, methods for manufacturing and processing the powders, granules and mini-tablets, and methods for treating cystic fibrosis employing the pharmaceutical composition.
Owner:VERTEX PHARMA INC
Pharmaceutical composition and administrations thereof
The present invention relates to pharmaceutical compositions containing a solid dispersion of N-[2,4-Bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide including formulations of the solid dispersions into powders, granules and mini-tablets, methods for manufacturing and processing the powders and mini-tablets and methods for treating cystic fibrosis employing the pharmaceutical composition.
Owner:VERTEX PHARMA INC
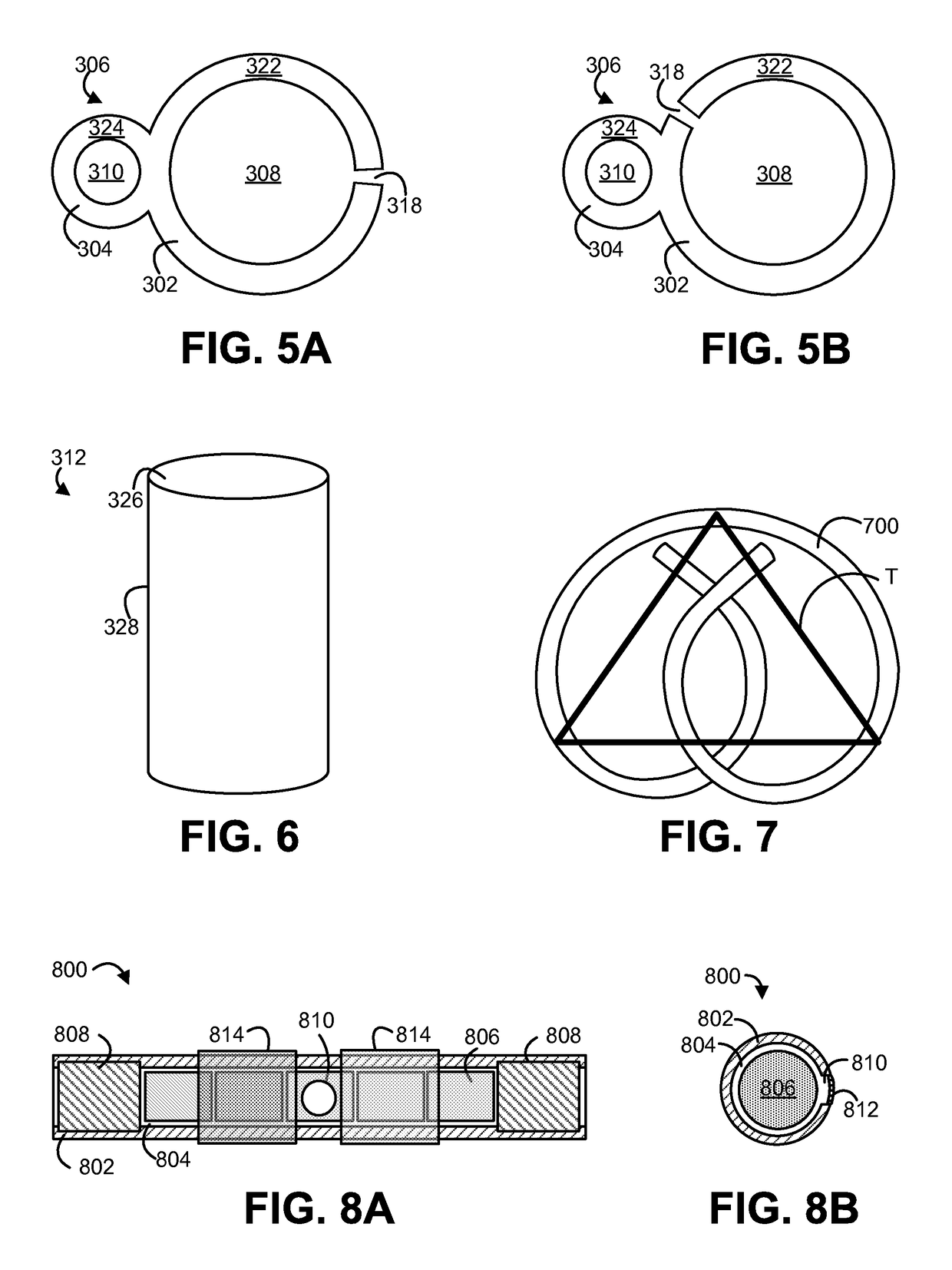
Physically/molecularly distributed and/or chemically bound medicaments in empty, hard capsule shells
The present invention incorporates medicaments in the empty hard capsule shells (body and cap). The medicament is either physically / molecularly distributed and / or chemically bound to the polymer matrix of the capsule shell composition. Other medicaments in the form of drug-loaded matrices (powders, granules, beads, pellets, mini-tablets, and mini-capsules) can be filled in the drug-loaded empty, hard capsule shells. The same capsule dosage form contains medicaments in the core matrix and in the shell.
Owner:JOSHI HEMANT N +1
Pharmaceutical composition and administrations thereof
The present invention relates to pharmaceutical compositions containing a solid dispersion of N-[2,4-Bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide including formulations of the solid dispersions into powders, granules and mini-tablets, methods for manufacturing and processing the powders, granules and mini-tablets, and methods for treating cystic fibrosis employing the pharmaceutical composition.
Owner:VERTEX PHARMA INC
Pharmaceutical Composition Comprising A Plurality of Mini-Tablets Comprising A Factor XA Inhibitor
A modified release pharmaceutical composition for oral administration comprising plural mini-tablets, comprising a therapeutically effective amount of a Factor Xa inhibitor within a matrix of polymer(s). The mini-tablets are suitably encapsulated within a gelatin capsule. A manufacturing process and method of use are also described.
Owner:GLAXO GROUP LTD
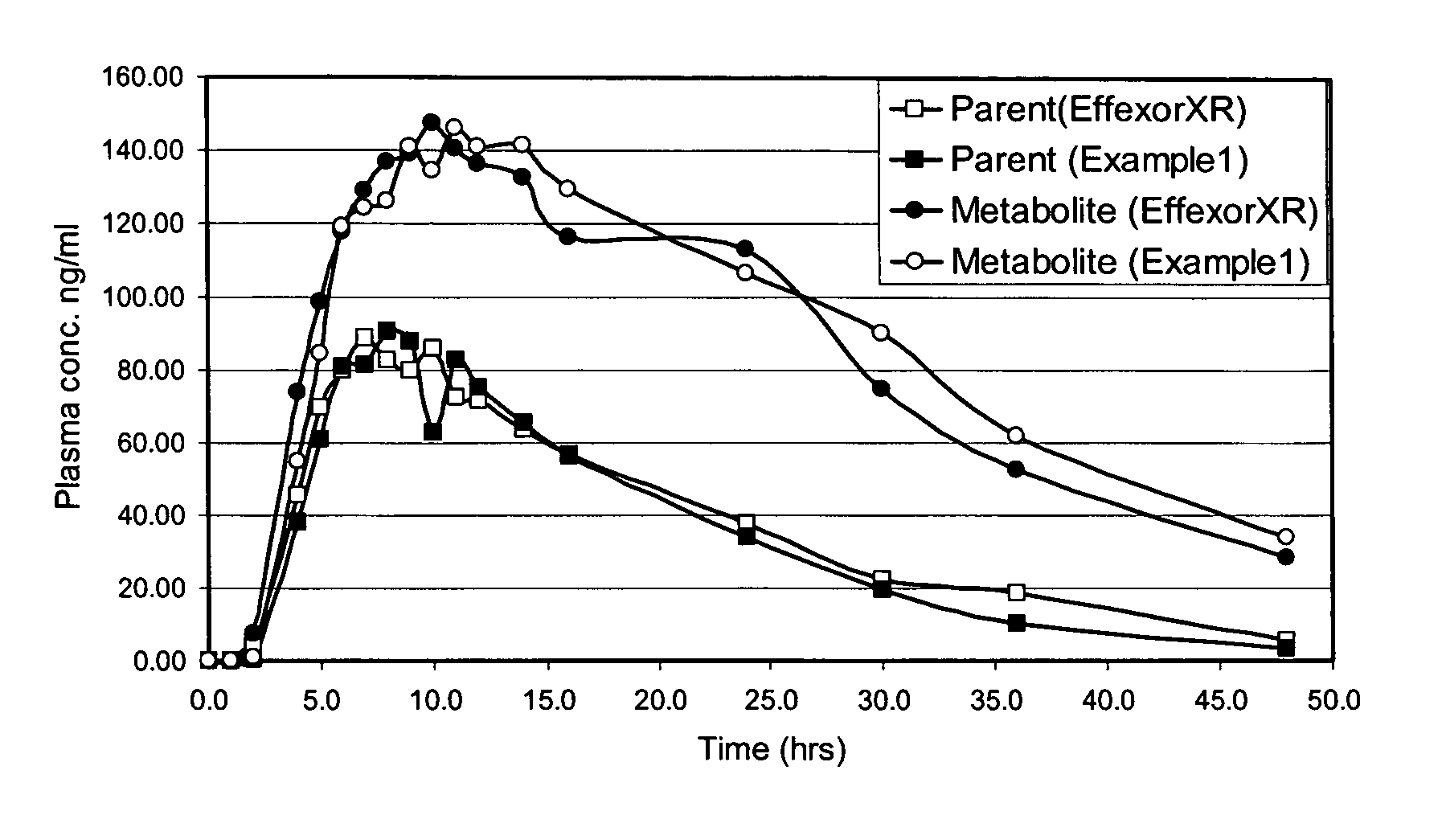
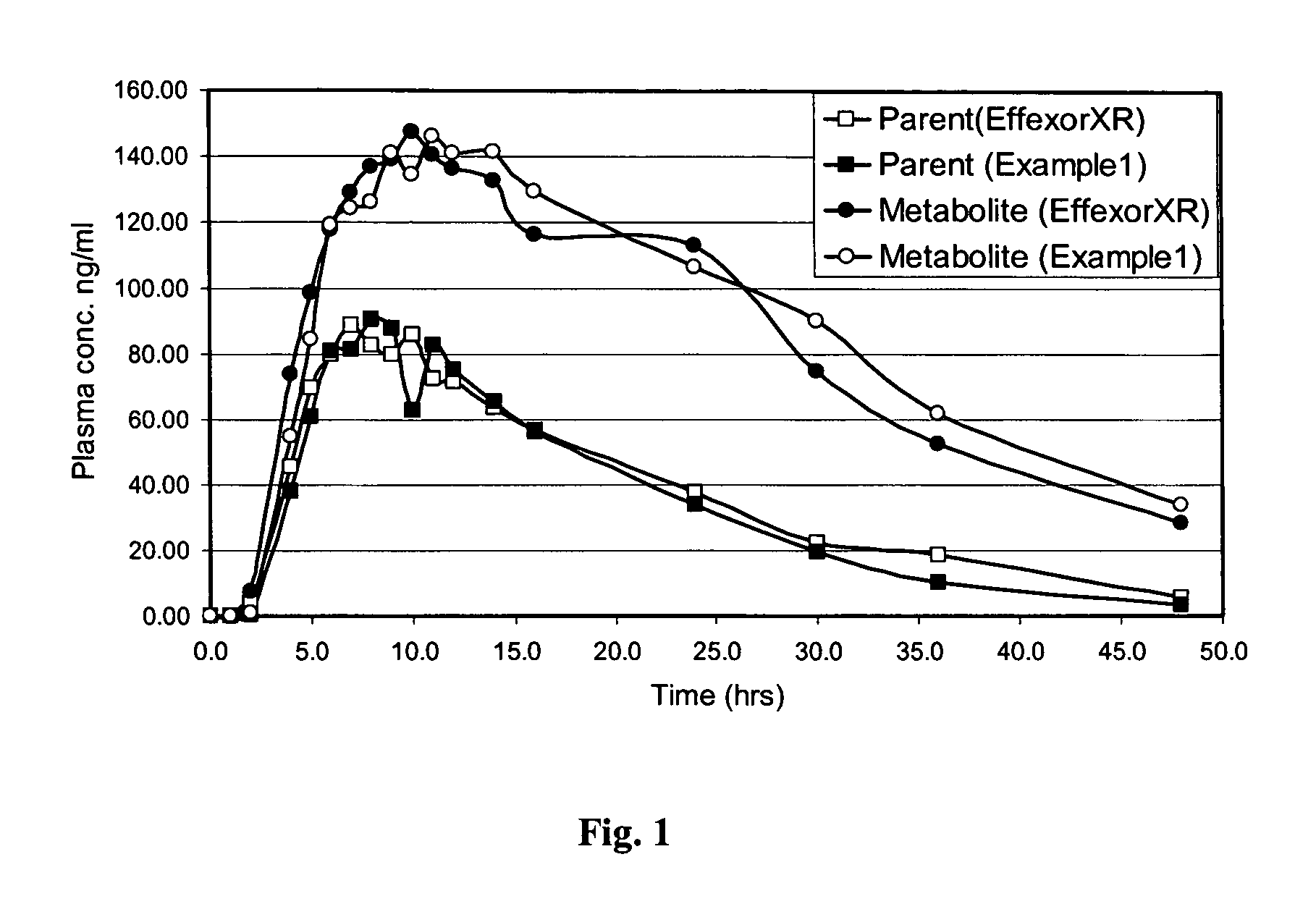
Extended release formulation of venlafaxine hydrochloride
InactiveUS20050169985A1Facilitated releaseReduce processing timeOrganic active ingredientsNervous disorderMini tabletsPharmaceutical formulation
The present invention relates to an extended release once daily pharmaceutical formulation comprising venlafaxine hydrochloride and pharmaceutically acceptable excipients. More particularly, the present invention relates to an extended release composition in the form of mini-tablets which are incorporated in hard gelatin capsules.
Owner:ALEMBIC LTD
A dosage form containing two or more active pharmaceutical ingredients in different physical forms
A dosage form for administration of two or more active pharmaceutical ingredients to a subject, comprising a first pharmaceutical composition comprising a first active pharmaceutical ingredient and optionally one or more pharmaceutically acceptable excipients in a first physical form selected from the group consisting of powder, granule, pellet, bead or mini-tablet form, and at least a second pharmaceutical composition comprising a second active pharmaceutical ingredient and optionally one or more pharmaceutically acceptable excipients in a second physical form selected from the group consisting of granule, pellet, bead, mini-tablet or tablet form, wherein the composition is characterised in that said first and second physical forms are selected to be different to minimise interactions between said first and second pharmaceutical compositions and to allow separation of said first and second pharmaceutical compositions for analysis on the basis of size difference.
Owner:ALPHAPHARM PTY LTD
Sutained release formulation for venlafaxine hydrochloride
InactiveUS20060182797A1Quick releaseOrganic active ingredientsNervous disorderSustained Release CapsuleMini tablets
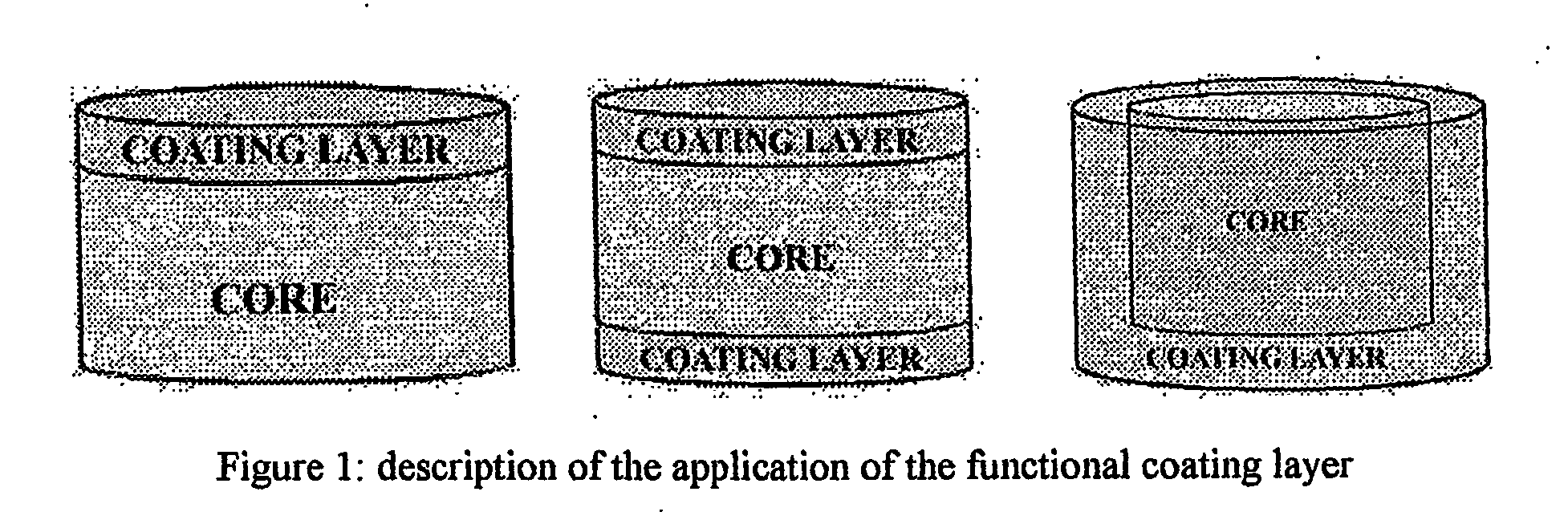
The invention provides a sustained release composition that; 1. Is free of initially increased drug delivery that occurs (in sustained release systems containing the water soluble drug venlafaxine HCl, known as burst phenomenon, by using a functional core partially or totally coated by a functional coating layer or film. 2. Delivers the drug substance within 24 hours and is therefore suitable for once daily administration of the said drug substance. 3. Exhibits linearity between the strength dosage form and the (total mass of the dosage form, by proportional increase of the amounts of the drug substance and the excipients in the formulation. 4. Is possible to be divided in smaller doses, without affecting the release of the drug substance. The invention provides a sustained release capsule formulation containing an appropriate number of functional complex mini tablets comprising of: I. A functional core comprising the active ingredient, especially the water-soluble drug Venlafaxine HCl and appropriate excipients. 2. A functional coating layer or film that reduces the initial surface of the core that is available for the release of the water-soluble drug Venlafaxine HClt phenomenon.
Owner:PHARMATHEN
Dosage Form Containing Two or More Active Pharmaceutical Ingredients in Different Physical Forms
ActiveUS20100092549A1Minimize interactionEasy to controlBiocideNervous disorderSize differenceMini tablets
A dosage form for administration of two or more active pharmaceutical ingredients to a subject, comprising a first pharmaceutical composition comprising a first active pharmaceutical ingredient and optionally one or more pharmaceutically acceptable excipients in a first physical form selected from the group consisting of powder, granule, pellet, bead or mini-tablet form, and at least a second pharmaceutical composition comprising a second active pharmaceutical ingredient and optionally one or more pharmaceutically acceptable excipients in a second physical form selected from the group consisting of granule, pellet, bead, mini-tablet or tablet form, wherein the composition is characterised in that said first and second physical forms are selected to be different to minimise interactions between said first and second pharmaceutical compositions and to allow separation of said first and second pharmaceutical compositions for analysis on the basis of size difference.
Owner:ALPHAPHARM PTY LTD
Anti-inflammatory pharmaceutical composition
InactiveUS20070224259A1BiocideElcosanoid active ingredientsMini tabletsNonsteroidal Antiinflammatory Drugs/NSAIDs
The present invention provides an orally administrable capsule comprising at least two mini-tablets, or at least two mini-capsules, or a combination of at least a mini-tablet and a mini-capsule, wherein one of said mini-tablet or mini-capsule comprises a nonsteroidal anti-inflammatory drug and the other mini-tablet or mini-capsule comprises a prostaglandin.
Owner:DR REDDYS LAB LTD +1
Dosage Form Having Polymorphic Stability
InactiveUS20070224260A1Improve liquidityBioavailability and impurity profilePowder deliveryGranular deliveryMini tabletsDosage form
Owner:DR REDDYS LAB LTD +1
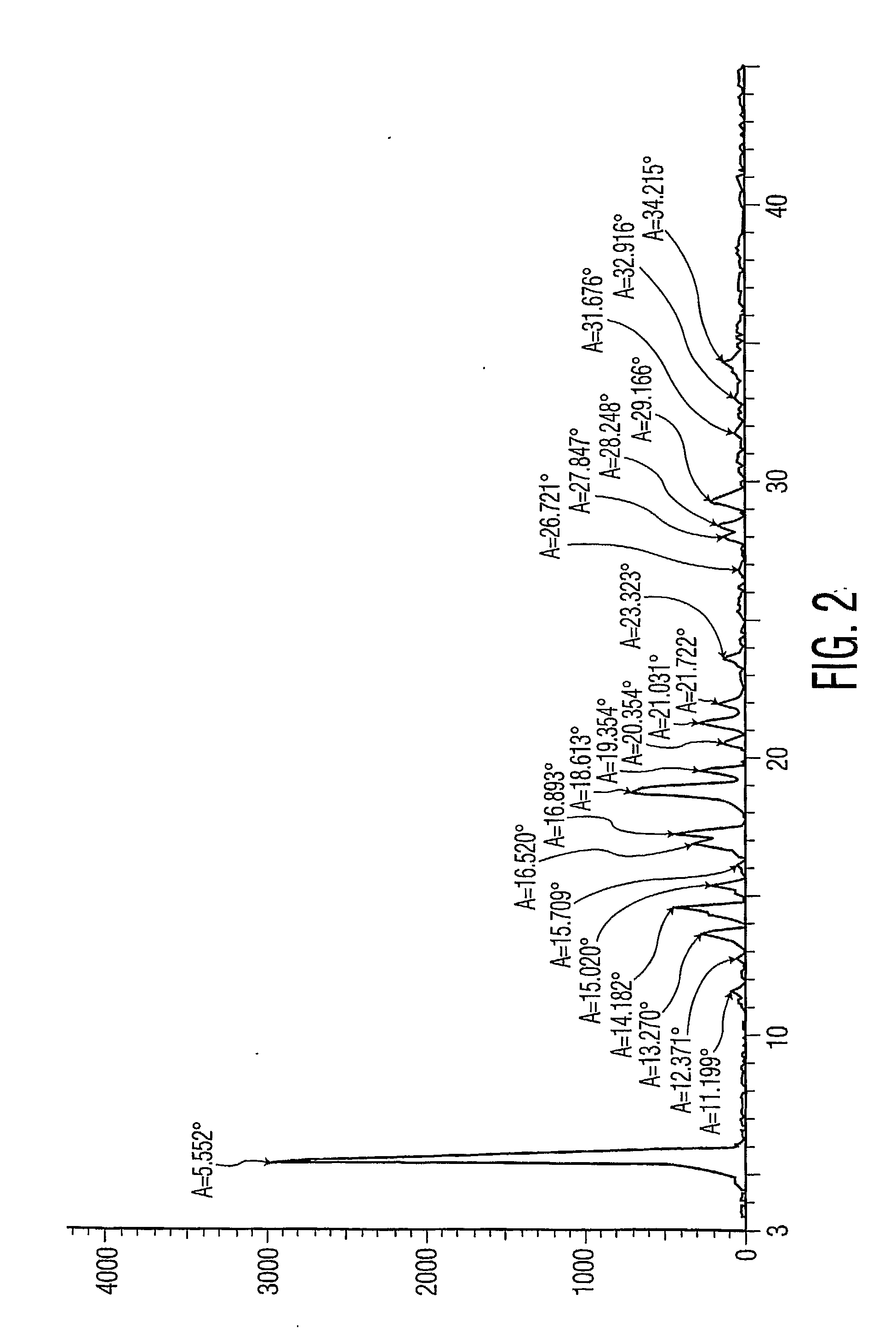
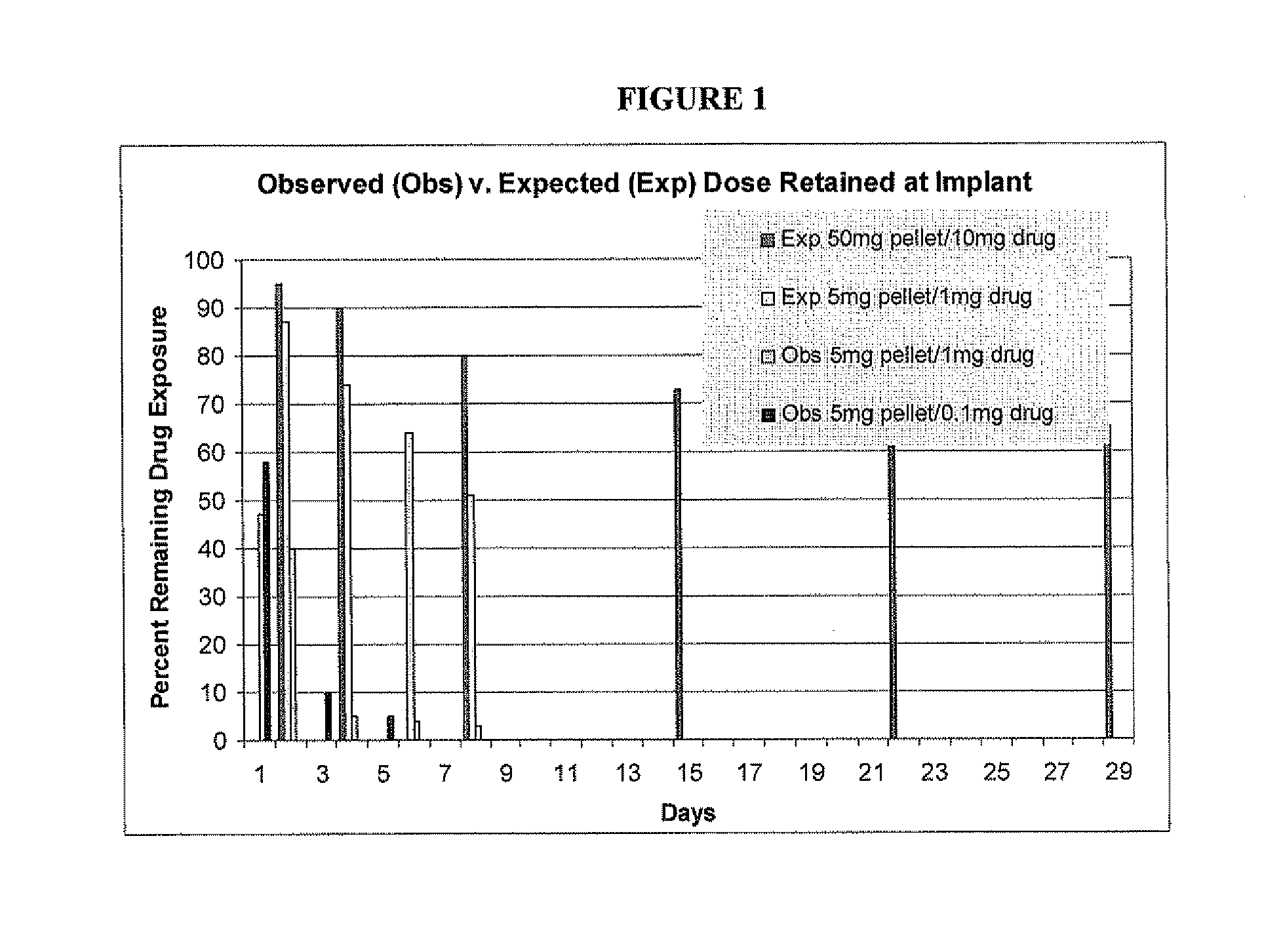
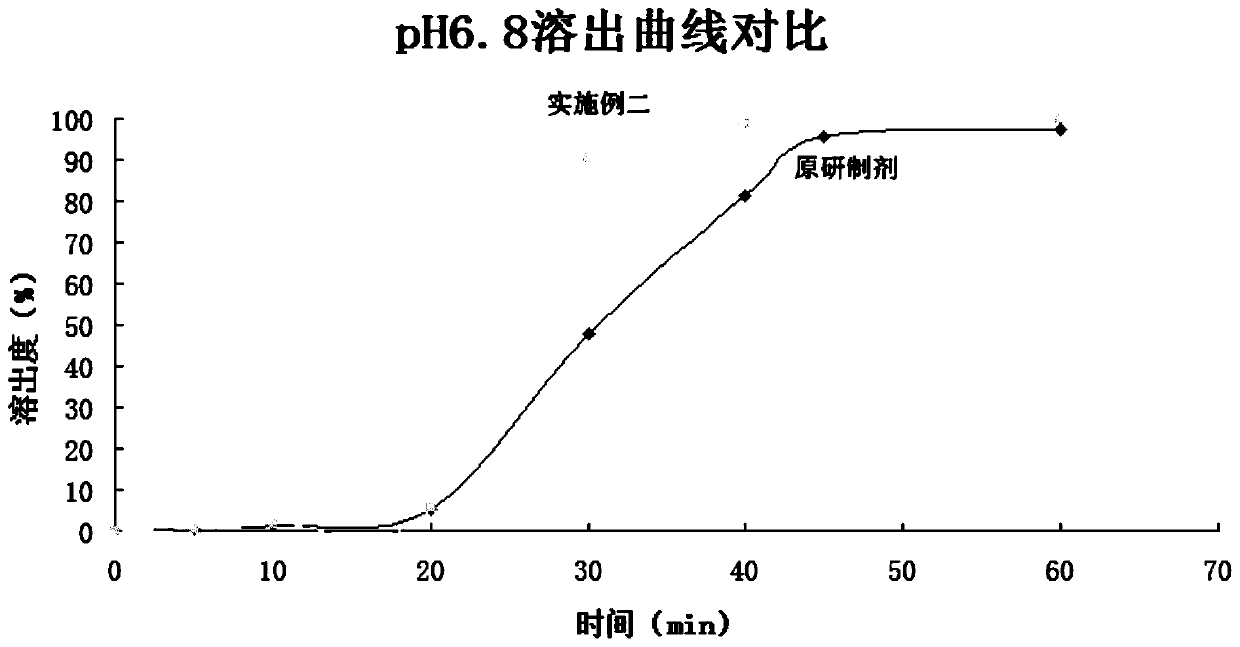
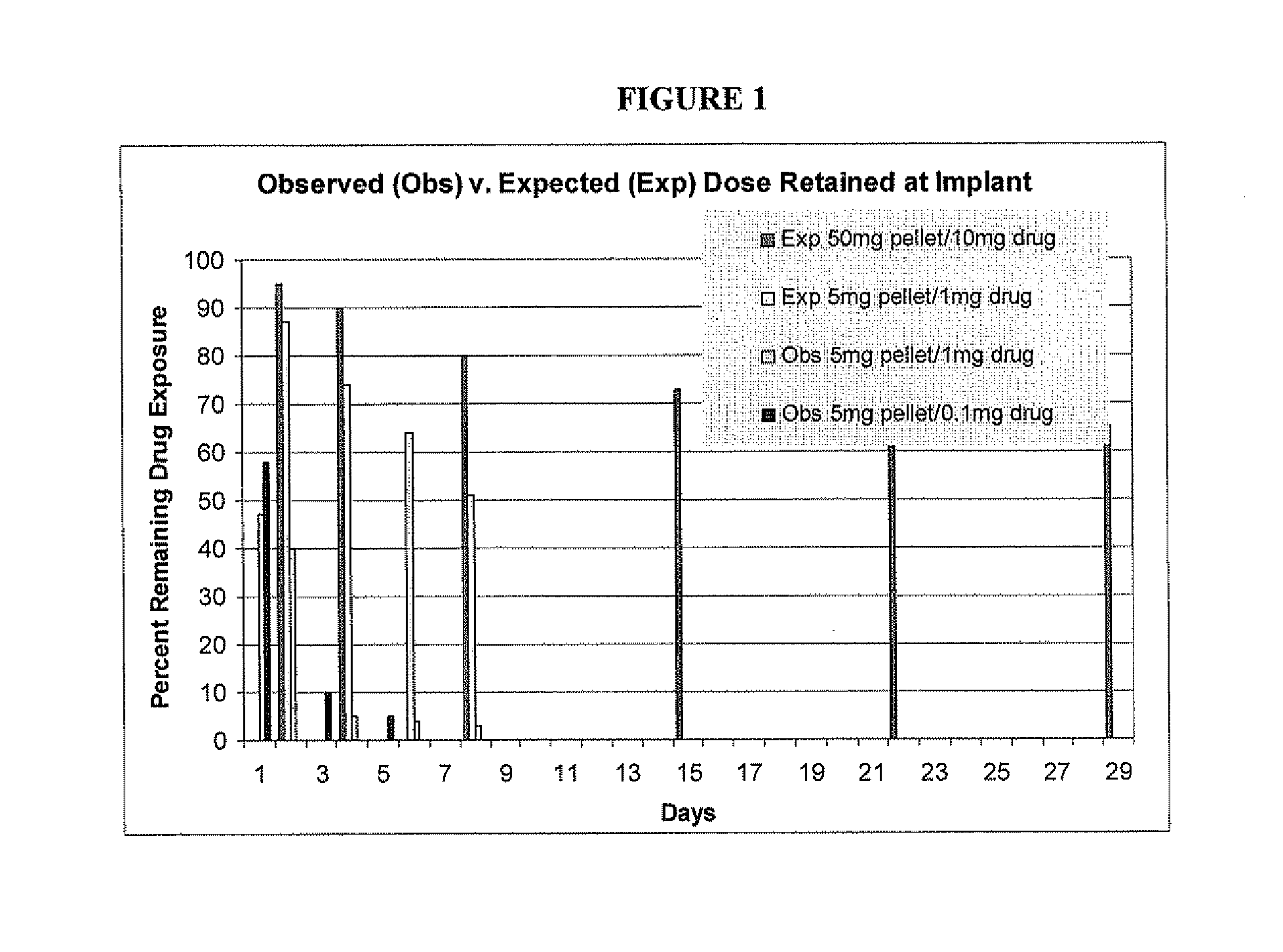
Rapid release mini-tablets provide analgesia in laboratory animals
ActiveUS20080096910A1Time-consuming to eliminateObviate expensiveBiocideNervous disorderPain therapyMedicine.hematology
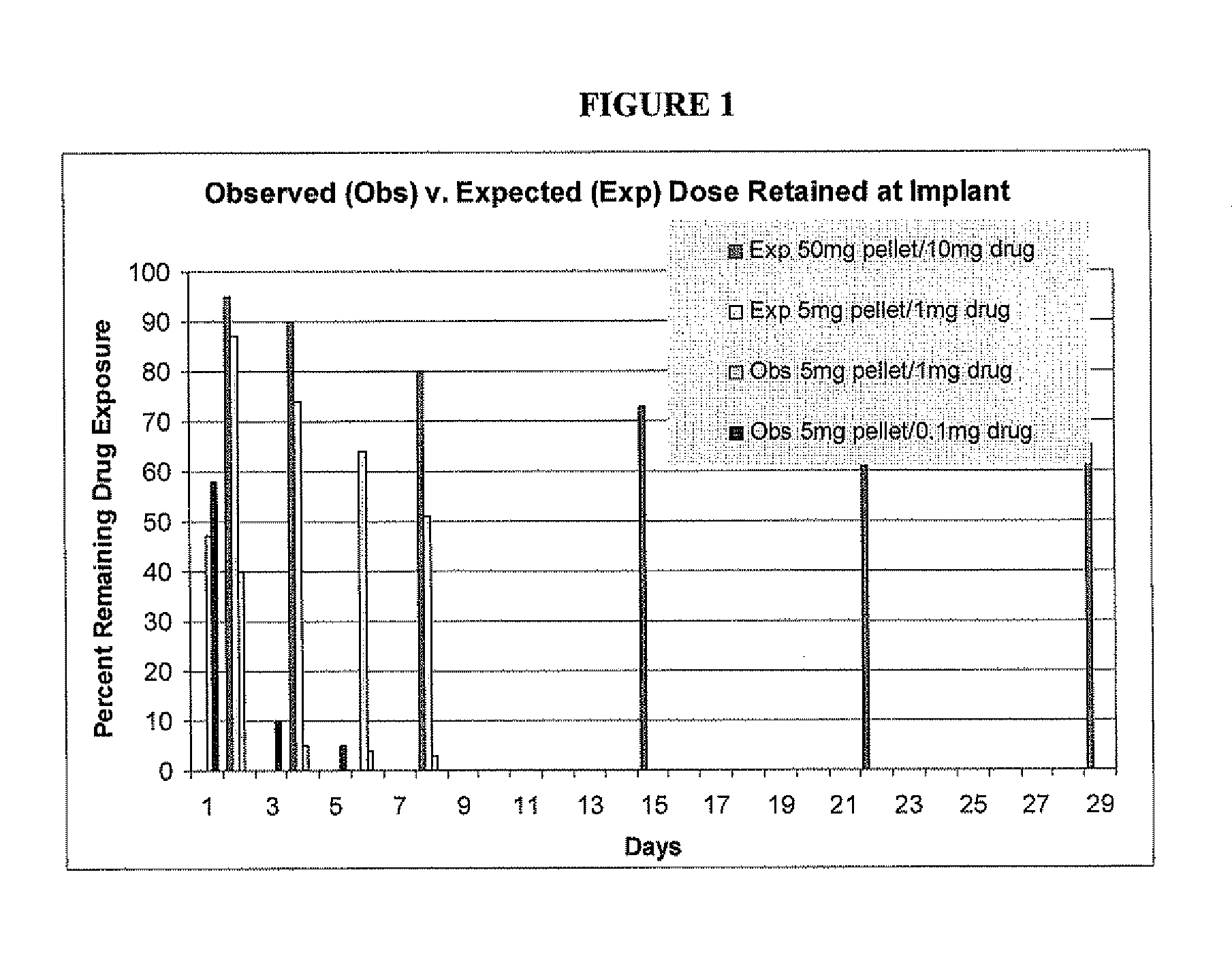
Pellets containing an analgesic uniformly dispersed in a lipid carrier such as cholesterol mixed with fatty acid esters, can be used to provide long term pain relief. 5 mg cholesterol-tryglyceride-buprenorphine pellets released the majority of drug in 24-48 hours after implant and provide clinically significant plasma levels of analgesia in mice for 3-9 days. Blood levels of analgesia peak at day-1 and are substantially complete by day-5 depending on the level of buprenorphine. These results demonstrate that post surgical implants provide clinically significant levels of analgesia in the 24-48 hour period following surgery and thus obviate the time consuming, expensive, and high-risk need to inject mice post surgery. The pellets are safe and easy to use. Placed in the surgical wound at the end of surgery, they provide 2-3 days of analgesia and obviate the need for subsequent handling of the animal for pain therapy. The implants have no detectable effect on mouse behavior, hematology, or liver chemistry. The unexpected release kinetics of the 5 mg pellet provides an ideal implant for post surgical analgesia. These implants solve a significant problem facing scientists who use rodents in research and abide by international of animal welfare.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Extended release formulation of venlafaxine hydrochloride
InactiveUS7807195B2Facilitated releaseReduce processing timeOrganic active ingredientsNervous disorderMini tabletsPharmaceutical formulation
The present invention relates to an extended release once daily pharmaceutical formulation comprising venlafaxine hydrochloride and pharmaceutically acceptable excipients. More particularly, the present invention relates to an extended release composition in the form of mini-tablets which are incorporated in hard gelatin capsules.
Owner:ALEMBIC LTD
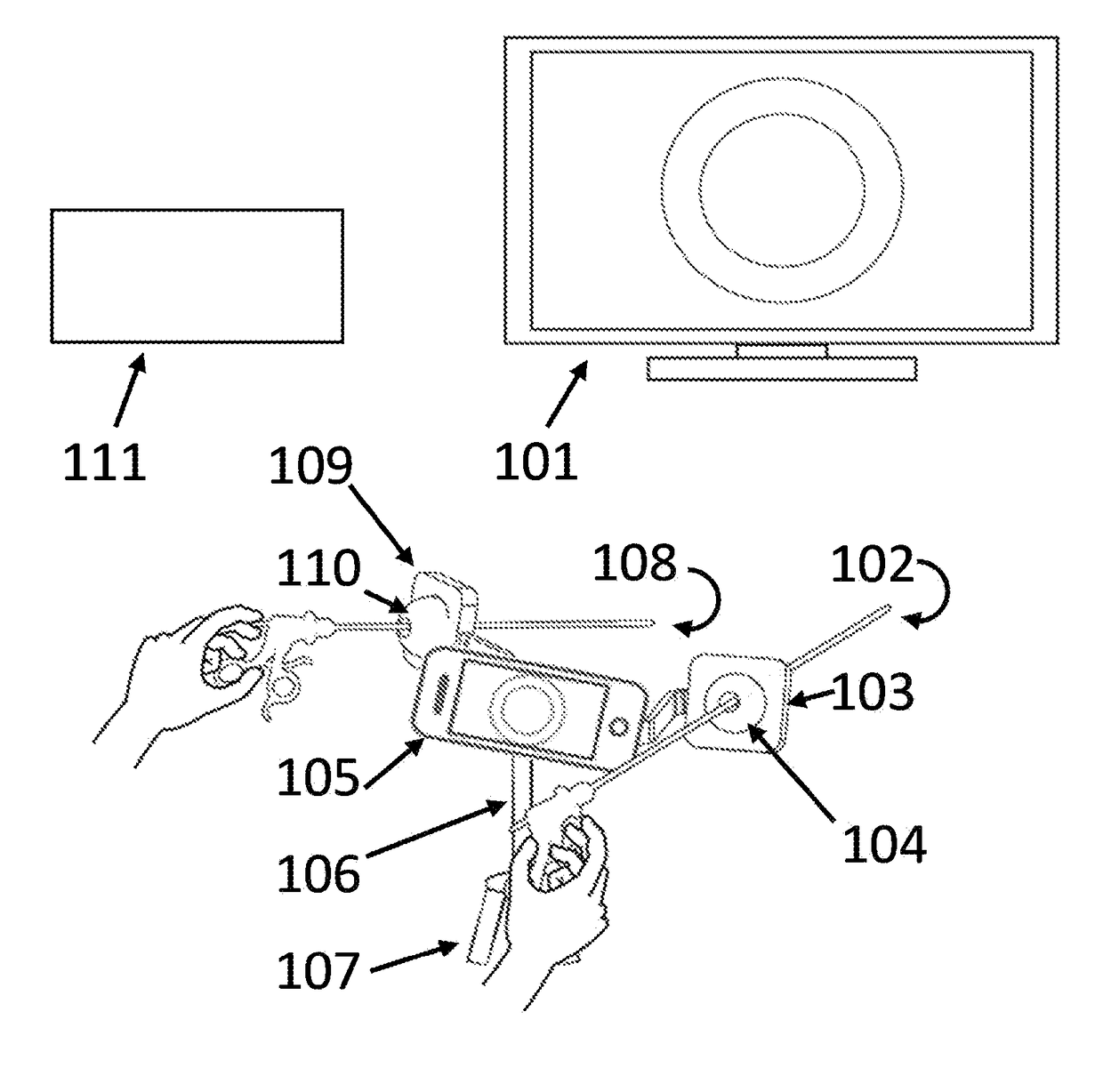
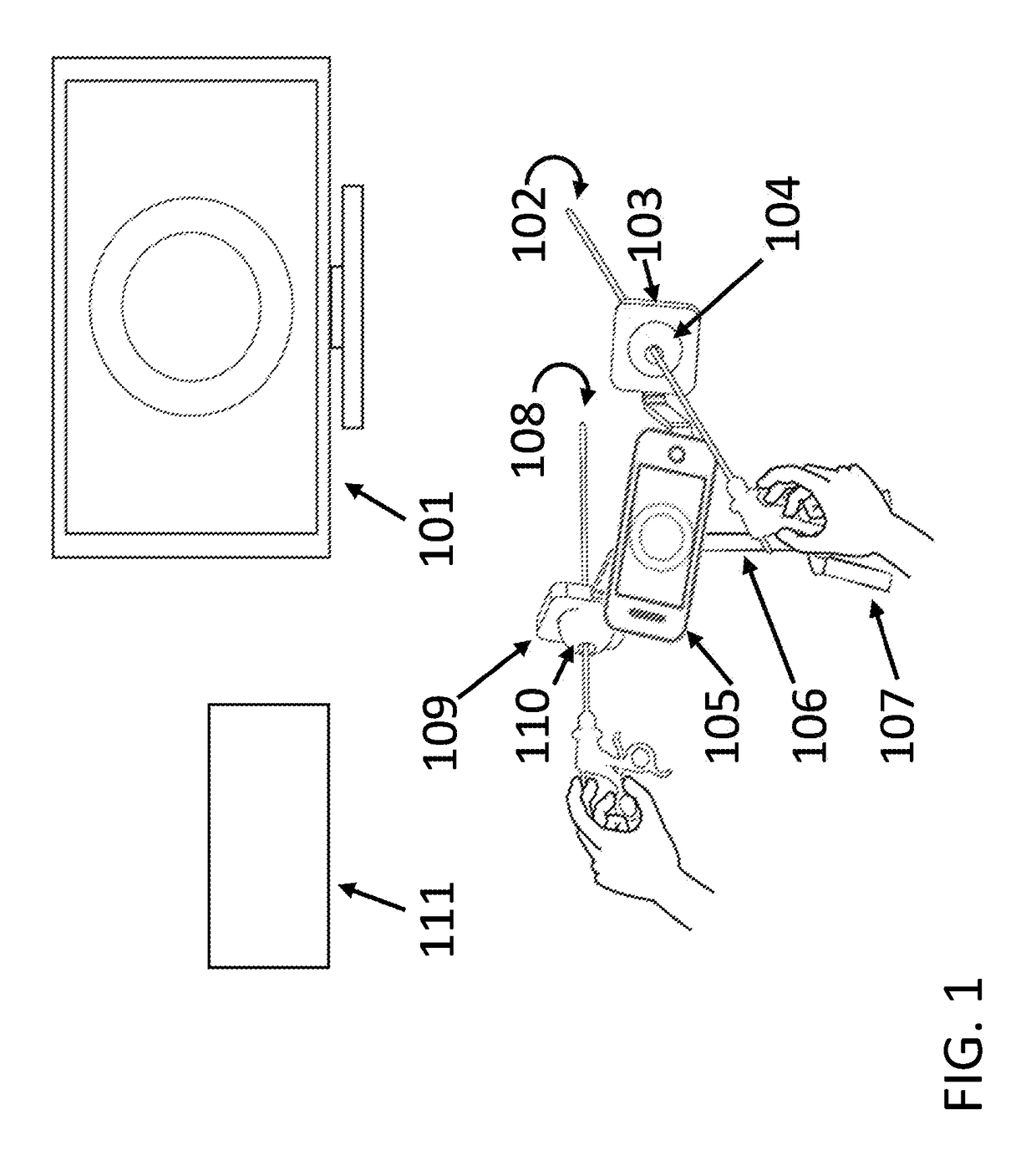
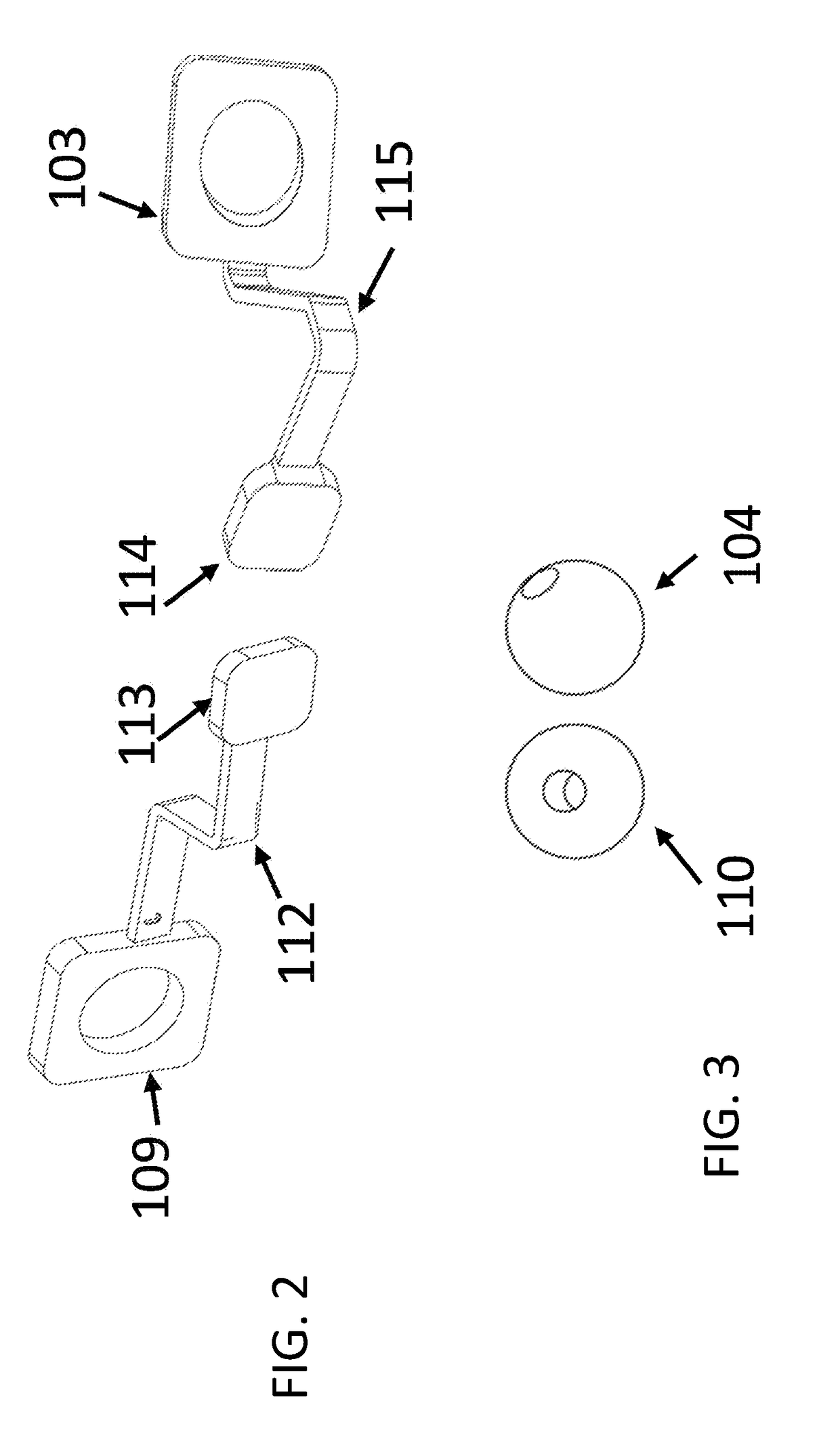
Laparoscopic Instrument Holders for Surgical SImulation and Training
InactiveUS20180005546A1Enhances surgical instrument rangeImprove realismEducational modelsTeaching apparatusMini tabletsPhysical model
Innovative instrument holders used for minimally invasive surgical simulation and training are disclosed when used in conjunction with a smartphone, tablet or mini-tablet computer enabling visualization of the surgical field. The surgical field used with these instrument holders can include animal models, physical models, and both virtual and augmented reality models. Some embodiments can be used with applications that can be downloaded to the smartphone, tablet or mini-tablet computer in order to enhance specific hand-eye coordination tasks. Some embodiments can be used as an adjunct surgical trainer for endoscopy, colonoscopy, and other minimally invasive gastrointestinal and gynecological surgical procedures using surgical instruments that incorporate fiber optics.
Owner:VAZQUEZ JOSE LUIS MOSSO
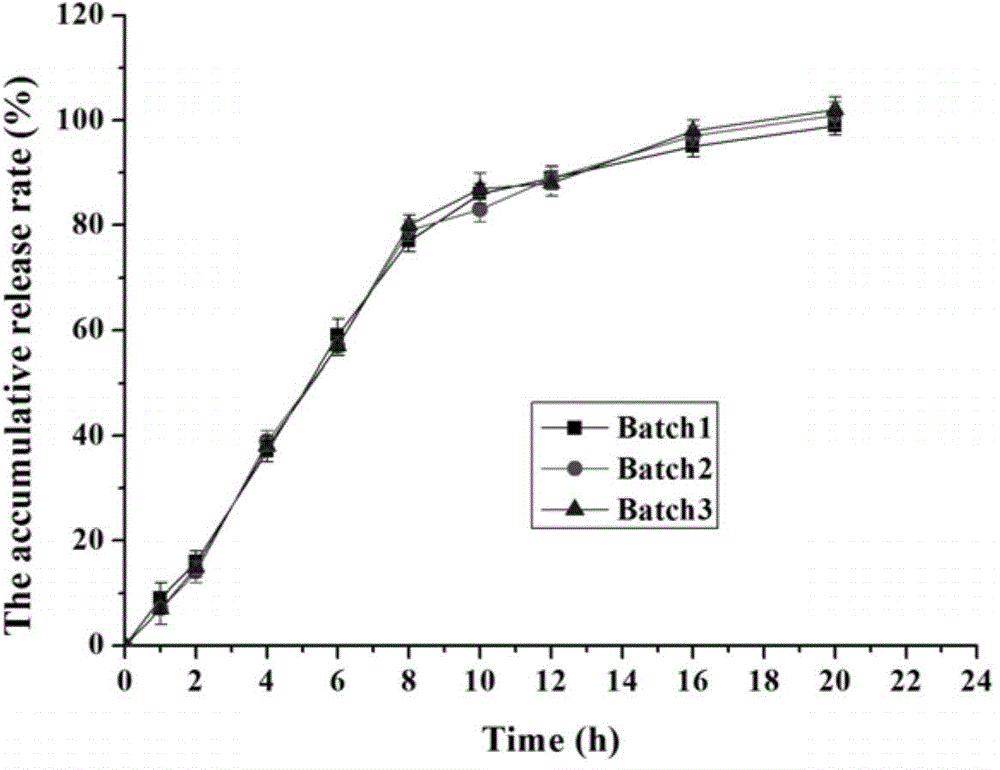
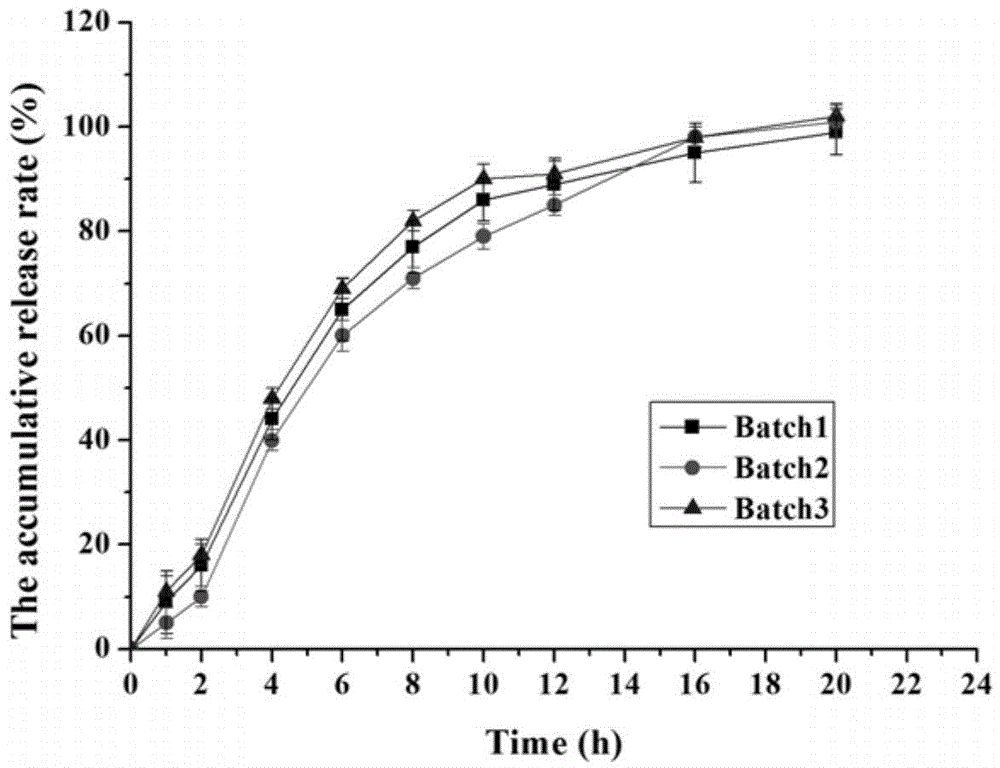
Memantine hydrochloride sustained release preparation and preparing method thereof
ActiveCN103816135ASimple production equipmentEase of industrial productionNervous disorderPharmaceutical delivery mechanismMemantine HydrochlorideMini tablets
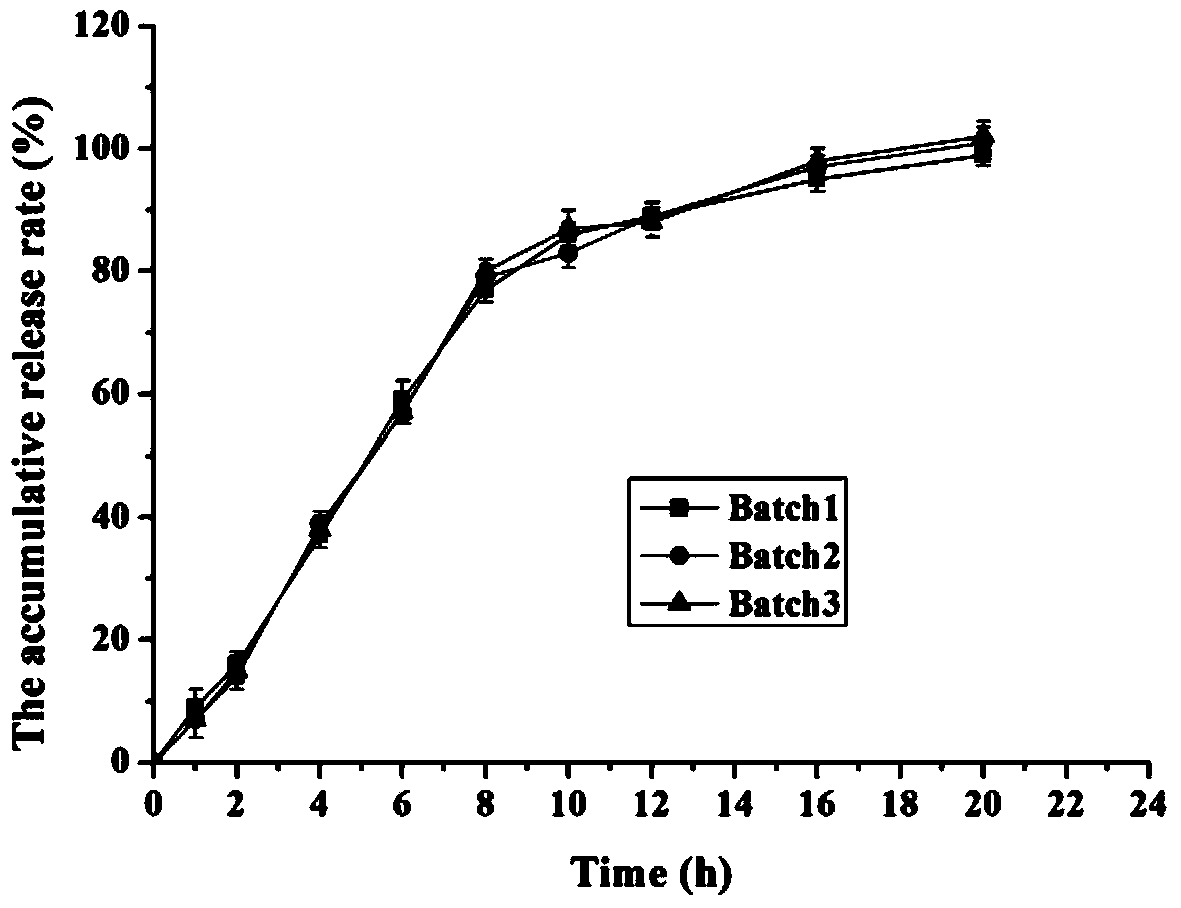
The invention discloses a memantine hydrochloride sustained release preparation. The memantine hydrochloride sustained release preparation comprises a memantine hydrochloride sustained release mini-tablet formed by a tablet core and a coating film. The weight of the coating film is 0-30% of the weight of the tablet core. Equipment for producing the sustained release mini-tablet is simple and is similar to equipment for producing common tablets, besides changes of the diameter size of a punch, and therefore industrial production of the sustained release mini-tablet can be easily achieved. After process parameters of the mini-tablet are determined, the sizes of obtained tablets are largely the same, in-batch differences or differences among batches are almost nonexistent, and the quality is easy to control.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
Physically/molecularly distributed and/or chemically bound medicaments in empty, hard capsule shells
The present invention incorporates medicaments in the empty hard capsule shells (body and cap). The medicament is either physically / molecularly distributed and / or chemically bound to the polymer matrix of the capsule shell composition. Other medicaments in the form of drug-loaded matrices (powders, granules, beads, pellets, mini-tablets, and mini-capsules) can be filled in the drug-loaded empty, hard capsule shells. The same capsule dosage form contains medicaments in the core matrix and in the shell.
Owner:JOSHI HEMANT N +1
Crystalline microspheres and the process for manufacturing the same
InactiveUS20150140097A1Reduce moisture absorption performanceReduce moisture contentPowder deliverySynthetic resin layered productsMicrosphereMini tablets
The present invention relates to microspheres and compositions comprising a plurality of microspheres, wherein the microspheres are perfectly spherical and have a moisture content less than 1%, and the method of manufacturing the same. The present invention is useful in the manufacture of sustained and modified release active pharmaceutical ingredient (API) microspheres, as a free flowing excipient for mini-tablets and in the manufacture of API dispersions.
Owner:SPI PHARMA
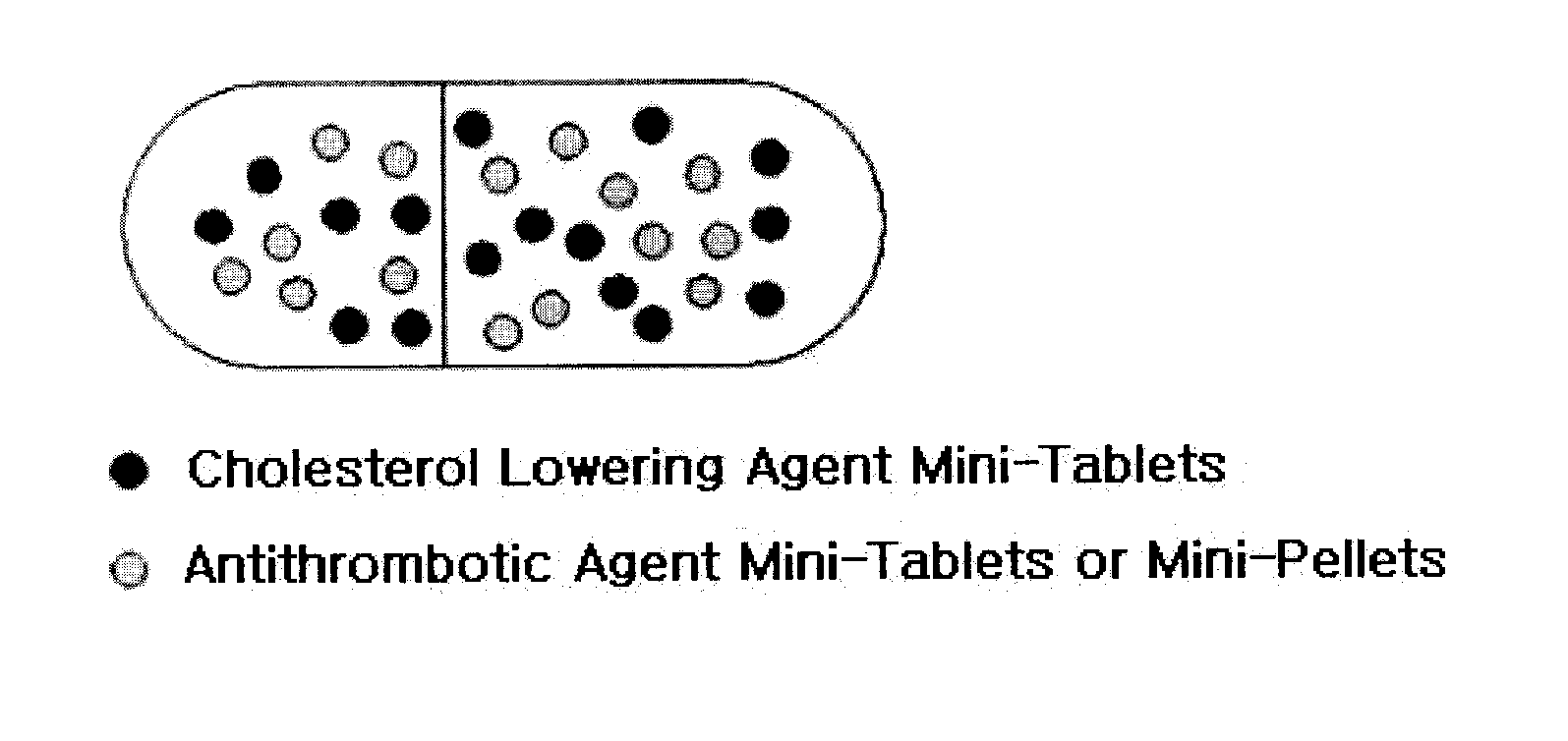
Combined formulation with improved stability
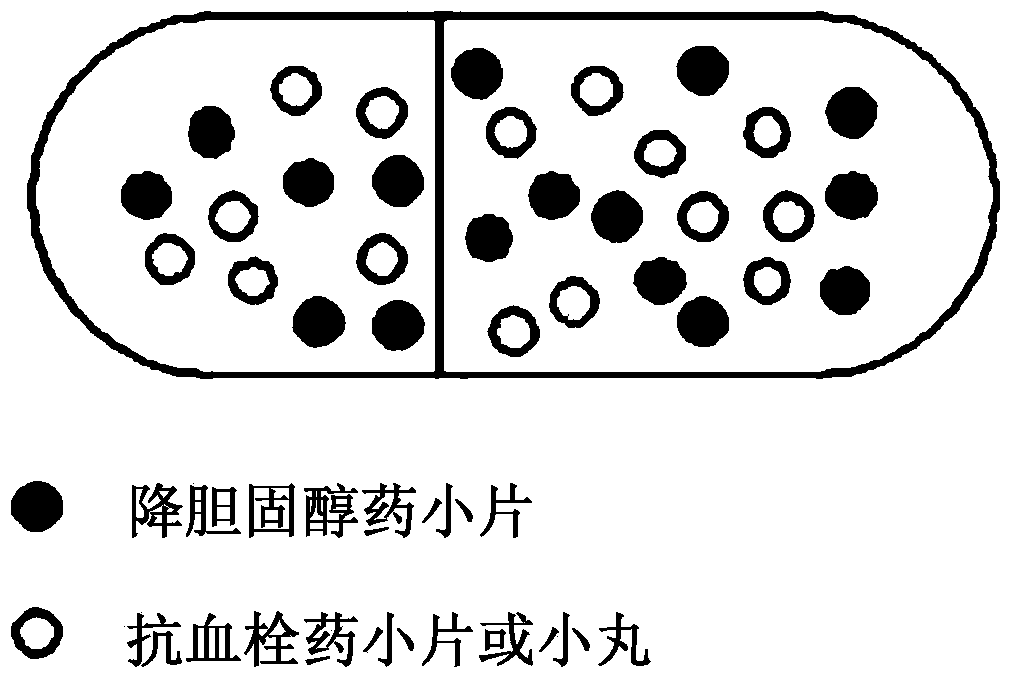
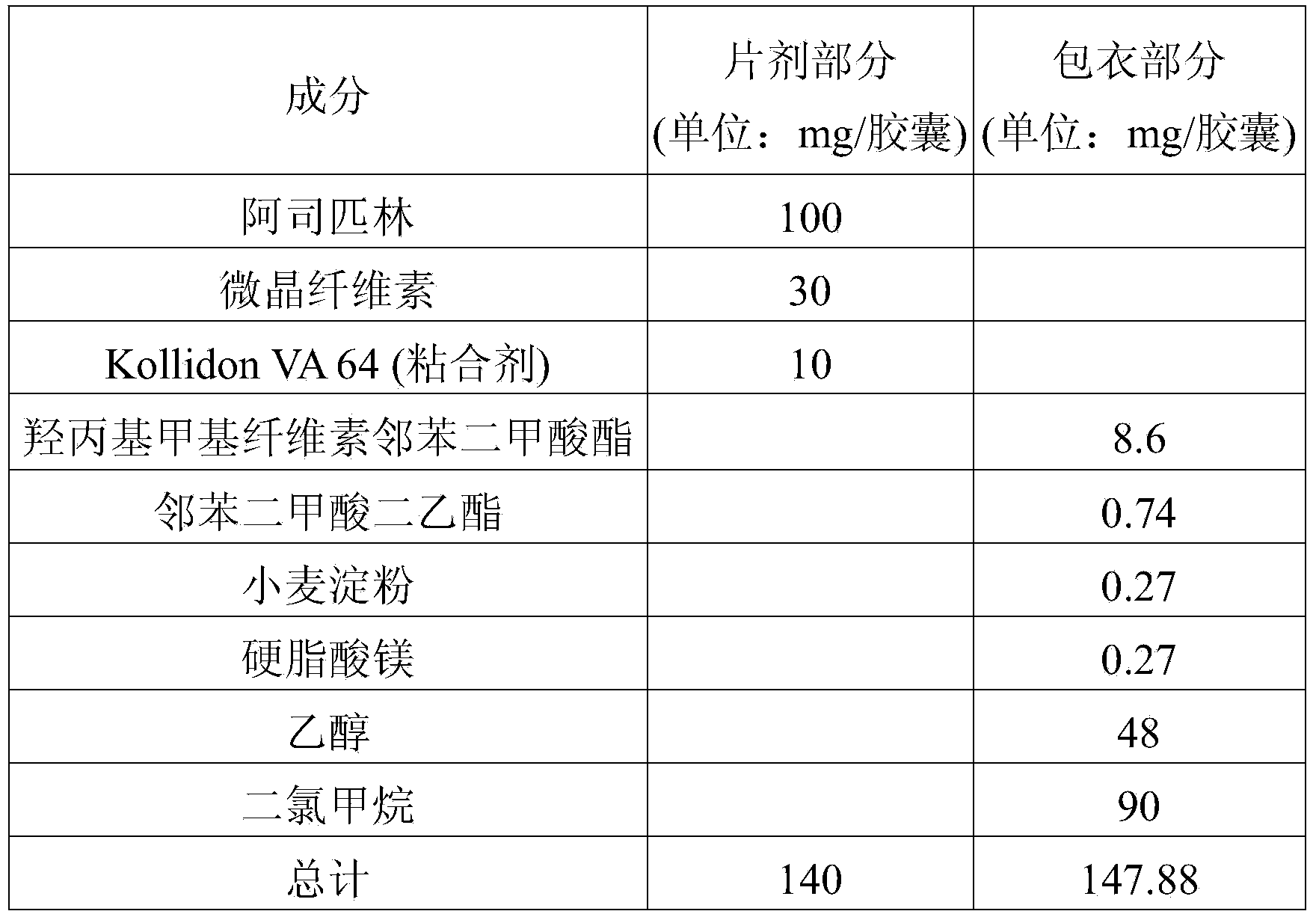
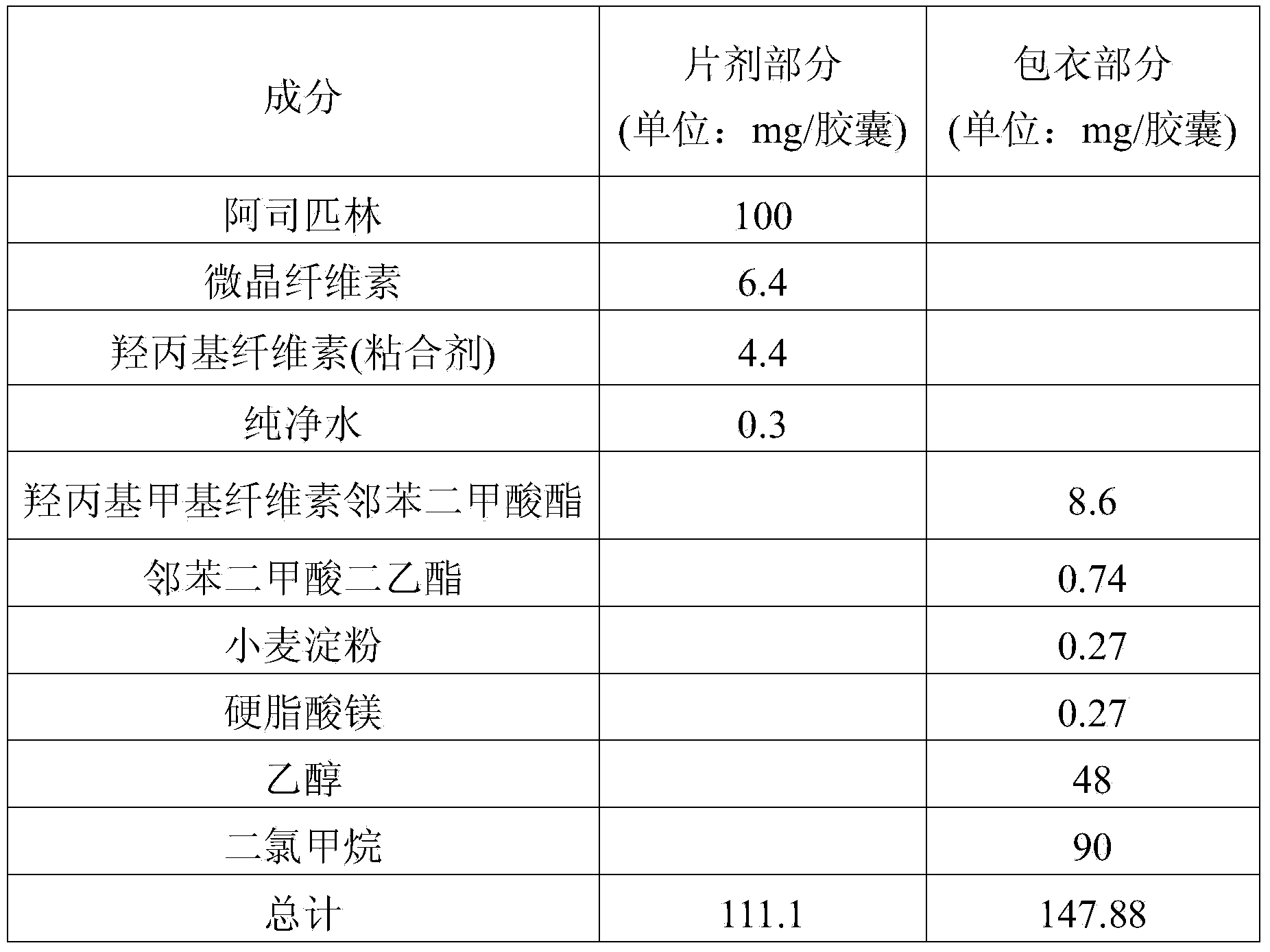
InactiveUS20140044784A1Reduce the amount requiredPrevent thrombosisBiocideAnimal repellantsAntithrombotic AgentSide effect
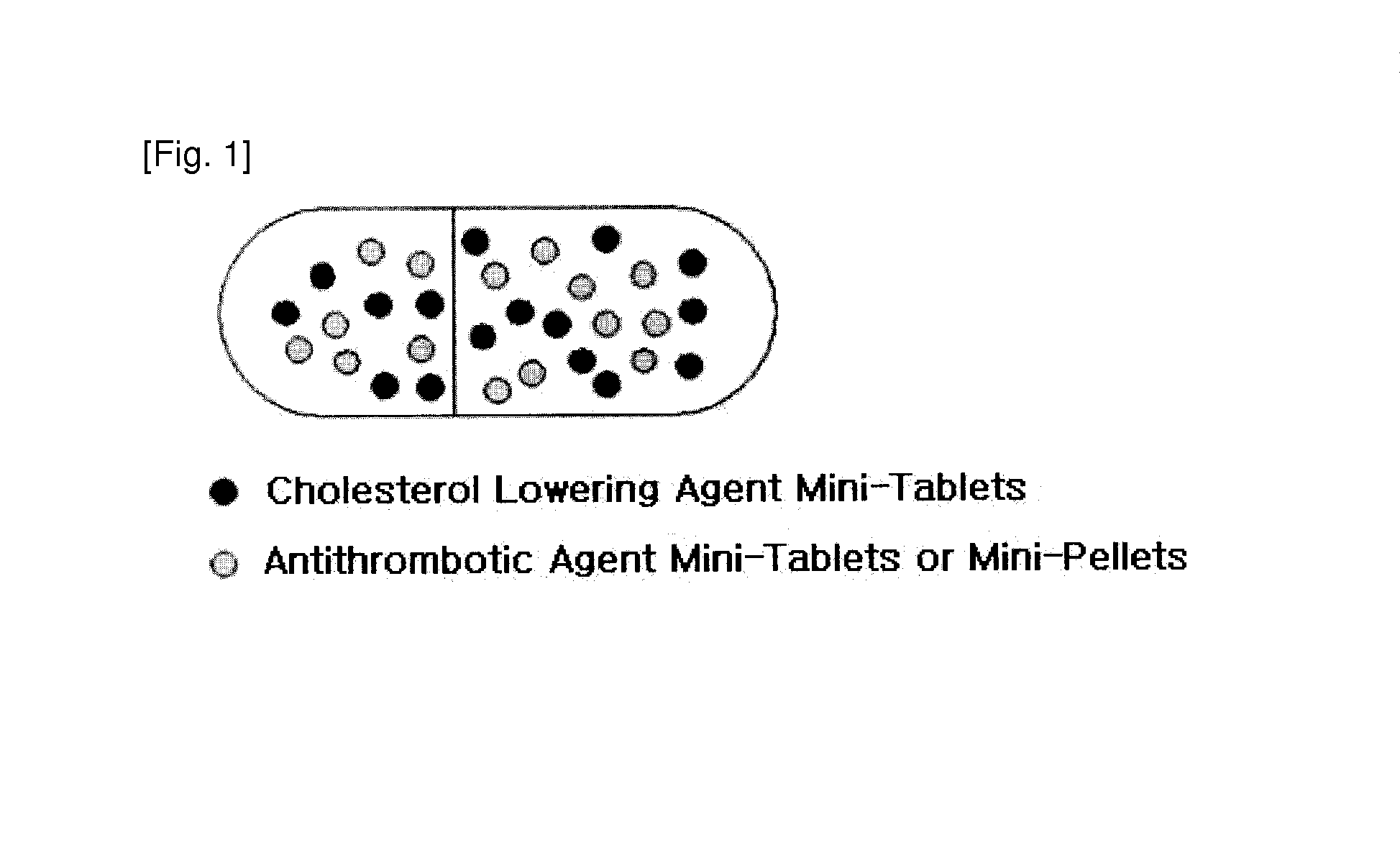
Disclosed is a combined formulation for oral administration to treat cardiovascular disease, including (a) cholesterol lowering agent mini-tablet having a diameter of 7.5 mm or less, which contain a cholesterol lowering agent, a stabilizer thereof and a pharmaceutically acceptable excipient and have a coating layer on the surface thereof, and (b) antithrombotic agent mini-tablets or mini-pellets having a diameter of 7.5 mm or less, which contain an antithrombotic agent and a pharmaceutically acceptable excipient and include an enteric coating film on the surface thereof. This formulation can improve treatment compliance depending on a combination prescription, and is controlled so that the cholesterol lowering agent is released in the gastrointestinal tracts and the antithrombotic agent is released in the intestines, thus suppressing the reactions and the side-effects between the drugs, inducing synergic effects of these drugs in vivo, and achieving improved stability.
Owner:BORYUNG PHARMA CO LTD
Crystalline microspheres and the process of manufacturing the same
ActiveUS20140099372A1Reduce moisture absorption performanceReduce moisture contentPowder deliveryGranular deliveryMicrosphereMini tablets
The present invention relates to microspheres comprising a core material, wherein the microsphere is perfectly spherical and has a moisture content less than 1%, and the method of manufacturing the same. The present invention is useful in the manufacture of sustained and modified release active pharmaceutical ingredient (API) microspheres, as a free flowing excipient for mini-tablets and in the manufacture of API dispersions.
Owner:SPI PHARMA
Combined formulation with improved stability
InactiveCN103533925AGood storage stabilityInhibit aggregationSalicyclic acid active ingredientsMetabolism disorderAntithrombotic AgentSide effect
Owner:BORYUNG PHARMA CO LTD
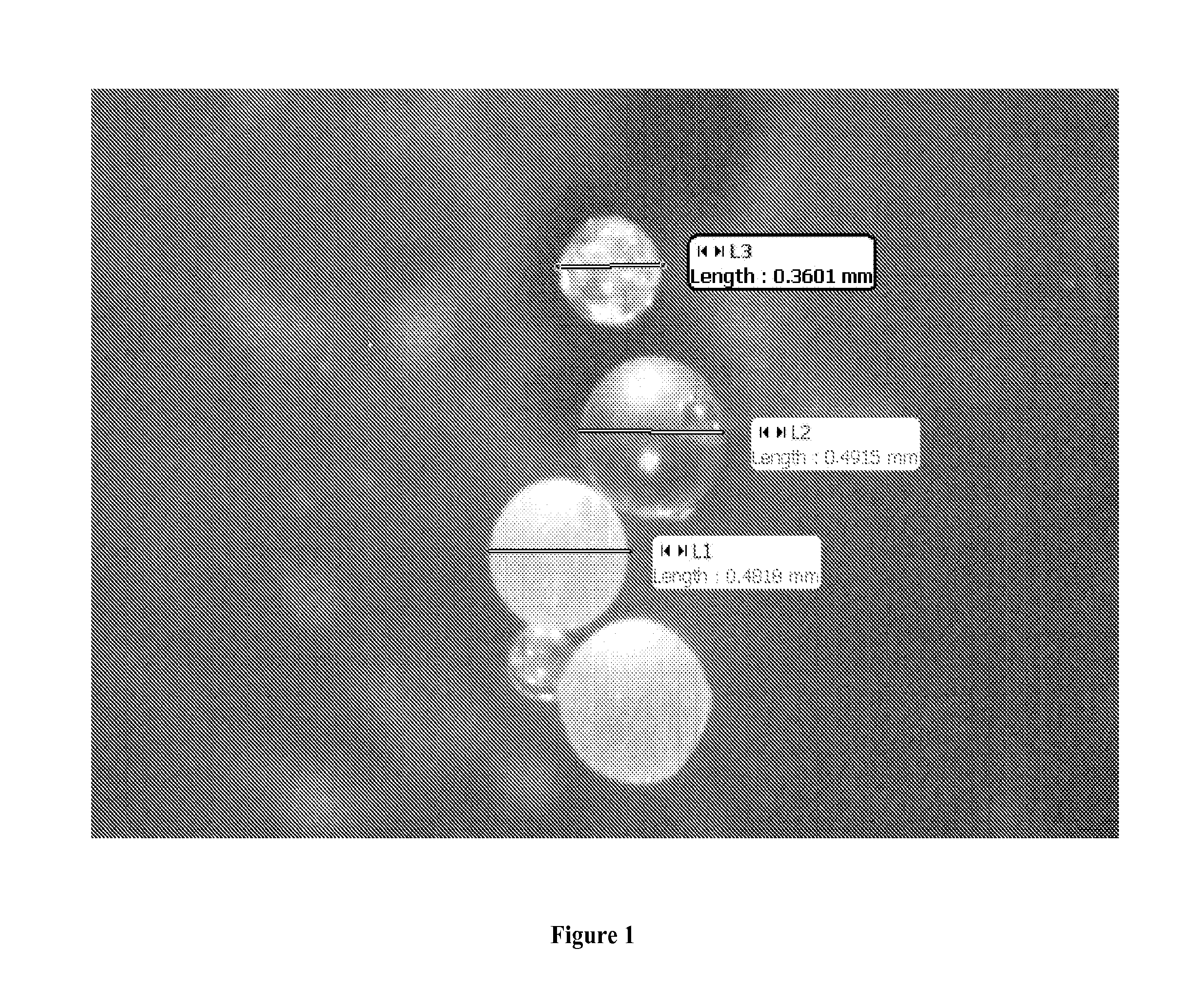
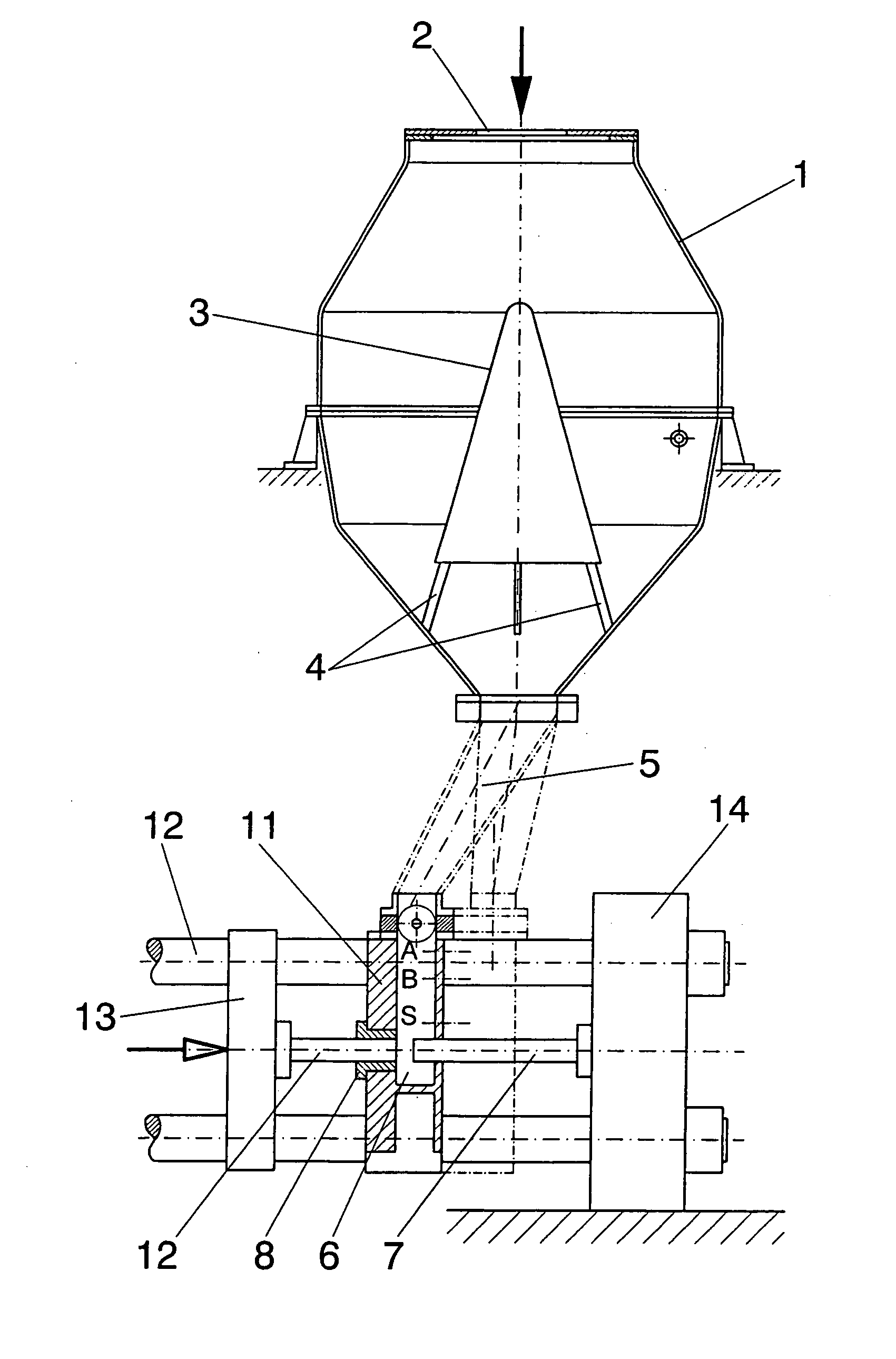
Method for the production of high-concentration manganese mini-tablets for alloying aluminum baths and device for implementing said method
The method has the aim of obtaining Mn mini-tablets with a concentration of the metal ranging between 90 and 98%, Al particles being the binding element. The method is based on the use of ground electrolytic Mn from shales with a chemical purity of 99.7% or higher. The product is screened with a mesh of less than 450 micra, wherein the fine powder content should be less than 15%. Moreover, atomized powder Al obtained by mechanical processes with a granulometry of between 100 and 800 micra and with over 80% of the powder being between 350 and 720 micra should be used in the method. The method is carried out in a device having a storage hopper (1), a diffuser (4) of the product in the hopper (1), a hopper for compacting and shaping the mini-tablets in molds (9) in combination with pressing punches (7 and 8) and with the aid of an alveolar and dosing valve (10) mounted between the feed chamber (5) and the compacting chamber (6).
Owner:BOSTLAN
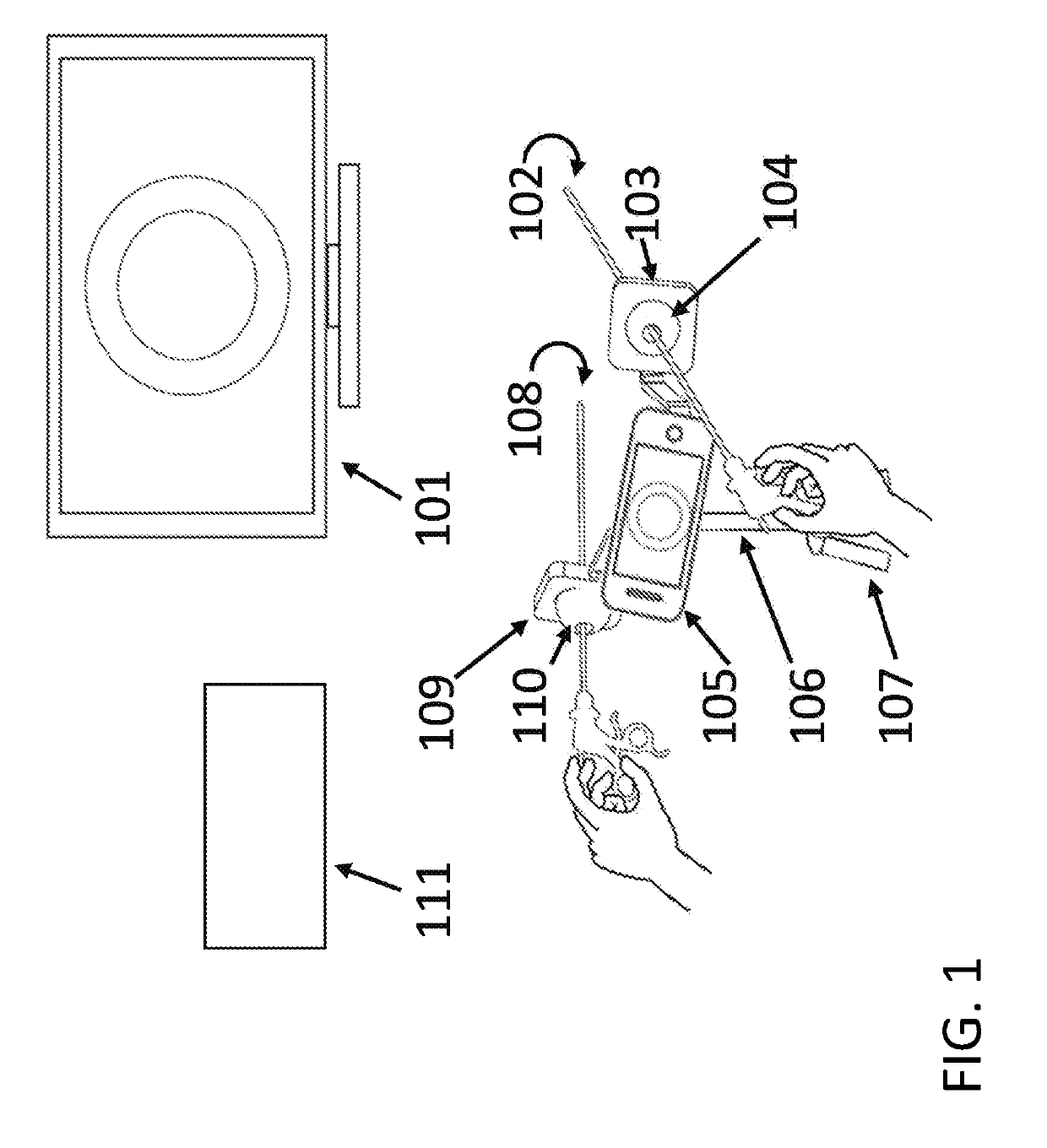
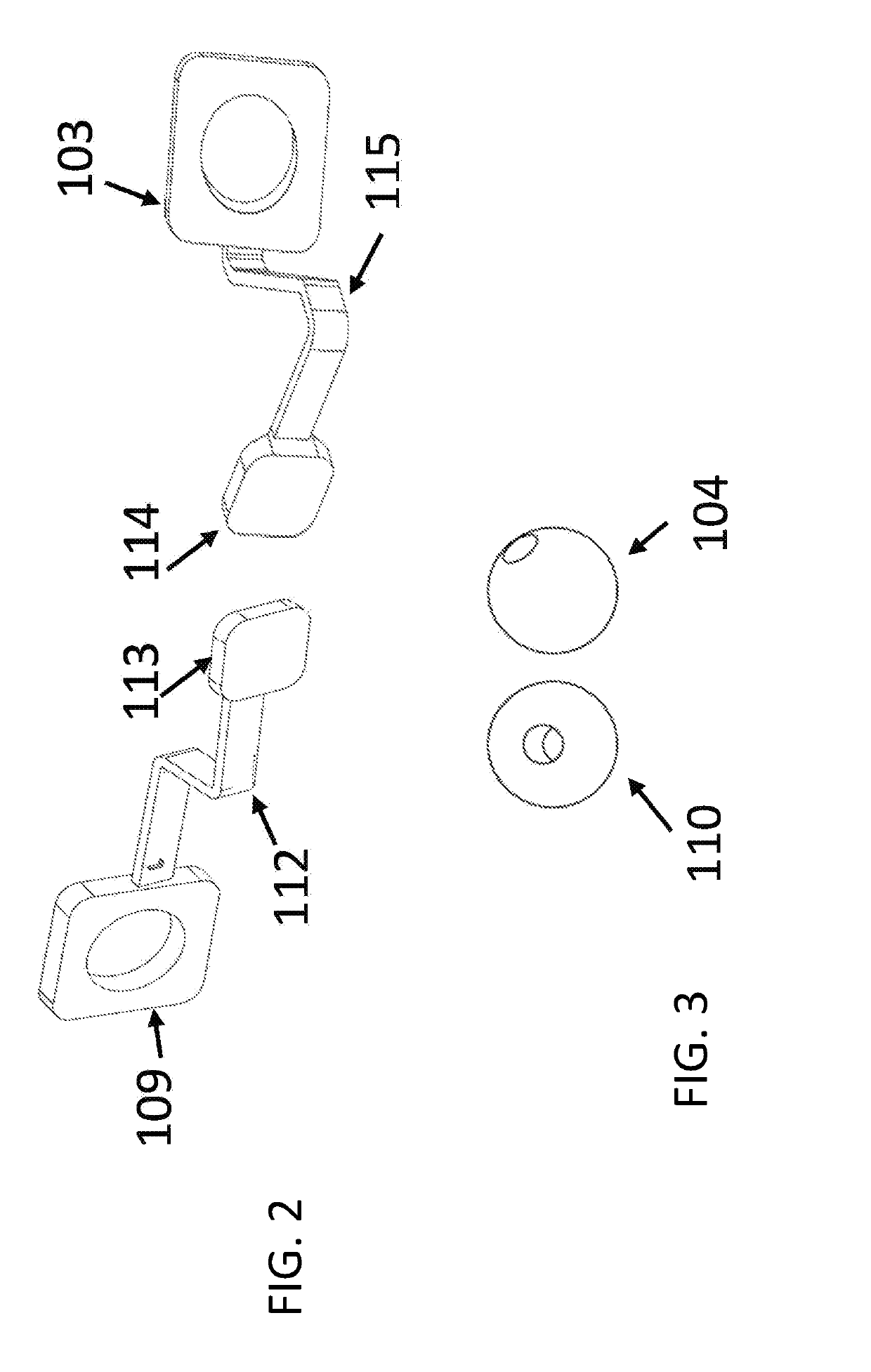
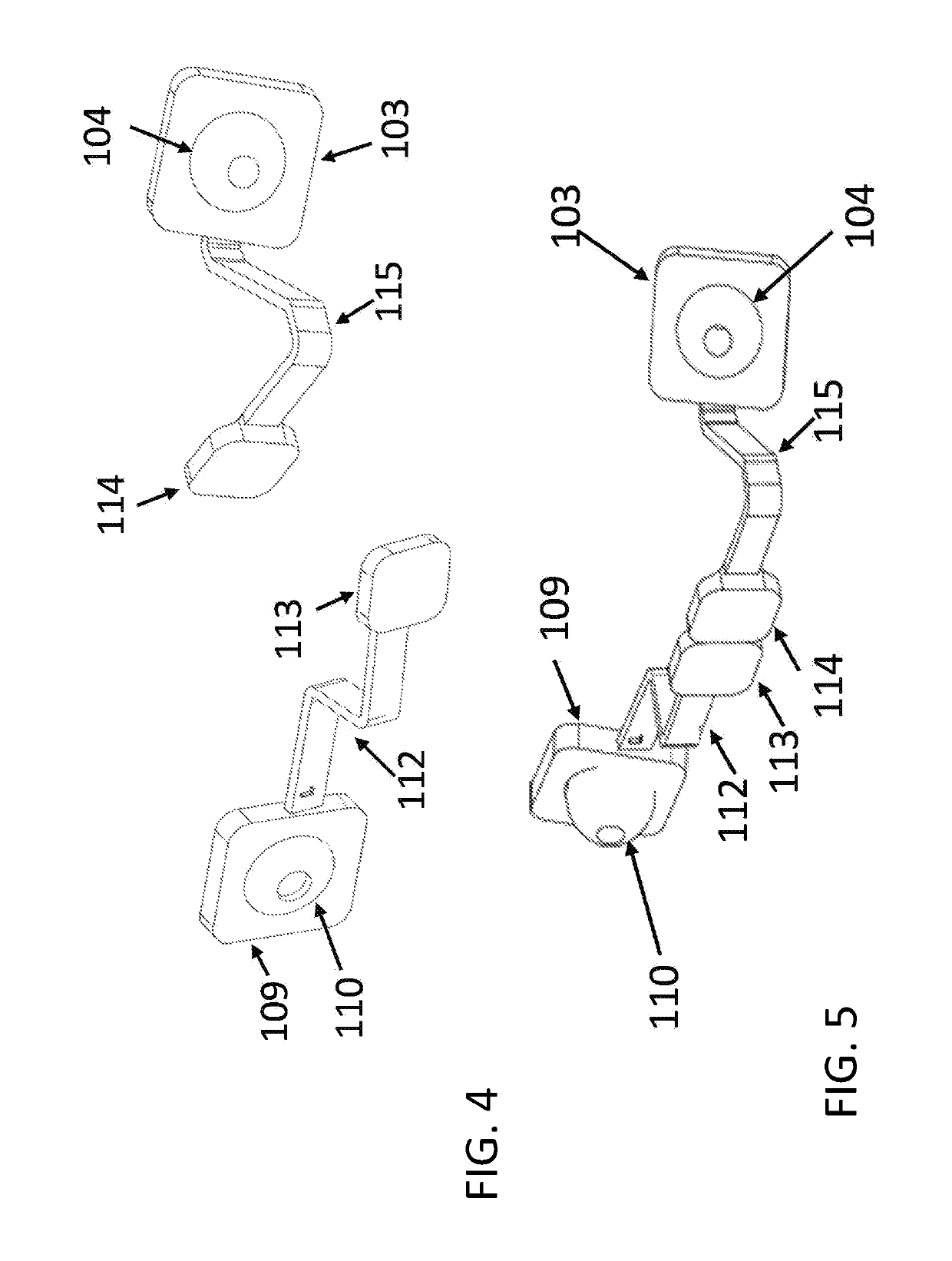
Solid drug tablets for implantable drug delivery devices
A drug dosage form is provided in the form of a solid tablet which is greater than 50% by weight the local anesthetic agent. The local anesthetic agent may be selected from the group consisting of an aminoamide, an aminoester, and a combination thereof. The drug tablet may be in the form of a mini-tablet which is greater than 70 wt % drug, with the balance being excipient. For example, the anesethetic agent may include lidocaine, in a salt or base form, combined with binder and lubricant excipients. Implantable drug delivery devices including the tablets are also provided, e.g., one or more of the drug tablets may be contained in a biocompatible housing. The drug tablets may be substantially cylindrical with flat end faces, and the device may have from 10 to 100 drug tablets aligned in the housing with the flat end faces of adjacent tablets abutting one another.
Owner:TARIS BIOMEDICAL
Memantine hydrochloride sustained-release preparation and preparation method thereof
ActiveCN103816135BSimple production equipmentEase of industrial productionNervous disorderPharmaceutical delivery mechanismMemantine HydrochlorideMini tablets
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
Rapid release mini-tablets provide analgesia in laboratory animals
ActiveUS20110287101A1Time-consuming to eliminateObviate expensiveBiocidePowder deliveryPain therapyMedicine.hematology
Pellets containing an analgesic uniformly dispersed in a lipid carrier such as cholesterol mixed with fatty acid esters, can be used to provide long term pain relief. 5 mg cholesterol-tryglyceride-buprenorphine pellets released the majority of drug in 24-48 hours after implant and provide clinically significant plasma levels of analgesia in mice for 3-9 days. Blood levels of analgesia peak at day-1 and are substantially complete by day-5 depending on the level of buprenorphine. These results demonstrate that post surgical implants provide clinically significant levels of analgesia in the 24-48 hour period following surgery and thus obviate the time consuming, expensive, and high-risk need to inject mice post surgery. The pellets are safe and easy to use. Placed in the surgical wound at the end of surgery, they provide 2-3 days of analgesia and obviate the need for subsequent handling of the animal for pain therapy. The implants have no detectable effect on mouse behavior, hematology, or liver chemistry. The unexpected release kinetics of the 5 mg pellet provides an ideal implant for post surgical analgesia. These implants solve a significant problem facing scientists who use rodents in research and abide by international of animal welfare.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Laparoscopic Instrument Holder for Surgical Simulation and Training
ActiveUS20190197919A1Increase freedomIncrease rangeEducational modelsTeaching apparatusFiberMini tablets
Owner:VAZQUEZ JOSE LUIS MOSSO
Pantoprazole sodium enteric mini-tablet capsule and preparation method thereof
InactiveCN110585166ASimple processSuccessful realization of industrial productionOrganic active ingredientsPharmaceutical non-active ingredientsMini tabletsMedicine
The invention belongs to the technical field of medicines, and particularly relates to a pantoprazole sodium enteric mini-tablet capsule and a preparation method thereof. The pantoprazole sodium enteric mini-tablet capsule comprises a pantoprazole sodium enteric mini-tablet placed in the capsule. The pantoprazole sodium enteric mini-tablet sequentially comprises a pantoprazole sodium tablet core,an isolation layer and an enteric layer from inside to outside. The pantoprazole sodium tablet core comprises the following components in percentage by mass: 20-60% of pantoprazole sodium, 10-46% of Pearlitol 200 SD, 15-20% of anhydrous sodium carbonate, 5-15% of PVPP, 3-5% of PVP.K30 and 0.5-2% of magnesium stearate. According to the invention, the stability of the product is improved, the bioavailability is basically consistent with that of reference listed drugs, and the acceleration stability is better than that of the reference listed drugs.
Owner:WUHAN RUNXIN TECH
Rapid release mini-tablets provide analgesia in laboratory animals
ActiveUS8093261B2Time-consuming to eliminateObviate expensiveBiocideNervous disorderPain therapyMini tablets
Pellets containing an analgesic uniformly dispersed in a lipid carrier such as cholesterol mixed with fatty acid esters, can be used to provide long term pain relief. 5 mg cholesterol-tryglyceride-buprenorphine pellets released the majority of drug in 24-48 hours after implant and provide clinically significant plasma levels of analgesia in mice for 3-9 days. Blood levels of analgesia peak at day-1 and are substantially complete by day-5 depending on the level of buprenorphine. These results demonstrate that post surgical implants provide clinically significant levels of analgesia in the 24-48 hour period following surgery and thus obviate the time consuming, expensive, and high-risk need to inject mice post surgery. The pellets are safe and easy to use. Placed in the surgical wound at the end of surgery, they provide 2-3 days of analgesia and obviate the need for subsequent handling of the animal for pain therapy. The implants have no detectable effect on mouse behavior, hematology, or liver chemistry. The unexpected release kinetics of the 5 mg pellet provides an ideal implant for post surgical analgesia. These implants solve a significant problem facing scientists who use rodents in research and abide by international of animal welfare.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Gastro-resistant enzyme pharmaceutical compositions
Owner:APTALIS PHARMA CANADA
Gastro-resistant enzyme pharmaceutical compositions
InactiveUS20130337062A1High drug contentA large amountNervous disorderPeptide/protein ingredientsMini tabletsMedicine
The present invention generally relates to compacted pharmaceutical compositions (such as tablets) comprising one or more enzymes, where the composition is monolithic or multiparticulates (such as mini-tablets, micro-tablets, or prills), or where the composition has multiple layers with the outermost layer containing one or more enzymes.
Owner:APTALIS PHARMA US +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com