A dosage form containing two or more active pharmaceutical ingredients in different physical forms
A technology of active pharmaceutical ingredients and physical forms, applied in the direction of organic active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve problems such as interference testing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Composition Weight / unit (mg)
[0084] Part A (powder)
[0085] Fluoxetine hydrochloride 27.95
[0086] Cornstarch 10.00
[0087] Pregelatinized corn starch 85.725
[0088] Magnesium stearate 1.325
[0089] Part B (small piece)
[0090] Olanzapine 6.00
[0091] Anhydrous lactose 47.15
[0092] Cornstarch 5.00
[0093] Pregelatinized Corn Starch 1.25
[0094] Crospovidone 2.00
[0095] Magnesium stearate 0.60
[0096] Total 187.00
[0097] The ingredients of Part A are suitably granulated and mixed as is known in the pharmaceutical formulation industry.
[0098] The ingredients of Part B are suitably granulated and mixed as is known in the pharmaceutical formulation industry. The resulting granules are compressed into tablets.
[0099] An appropriate amount of granules providing a sufficient amount of fluoxetine hydrochloride is filled into an appropriate size capsule and added to the olanzapine tablet.
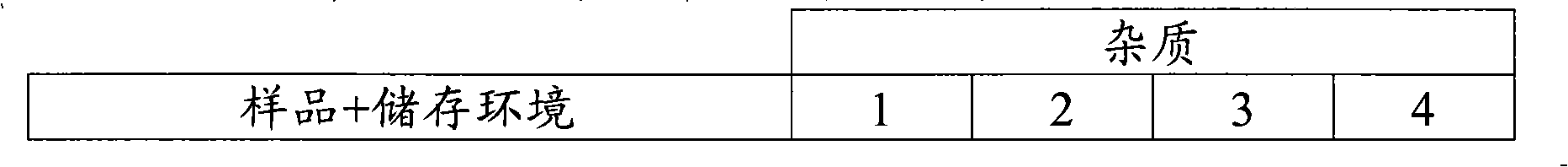
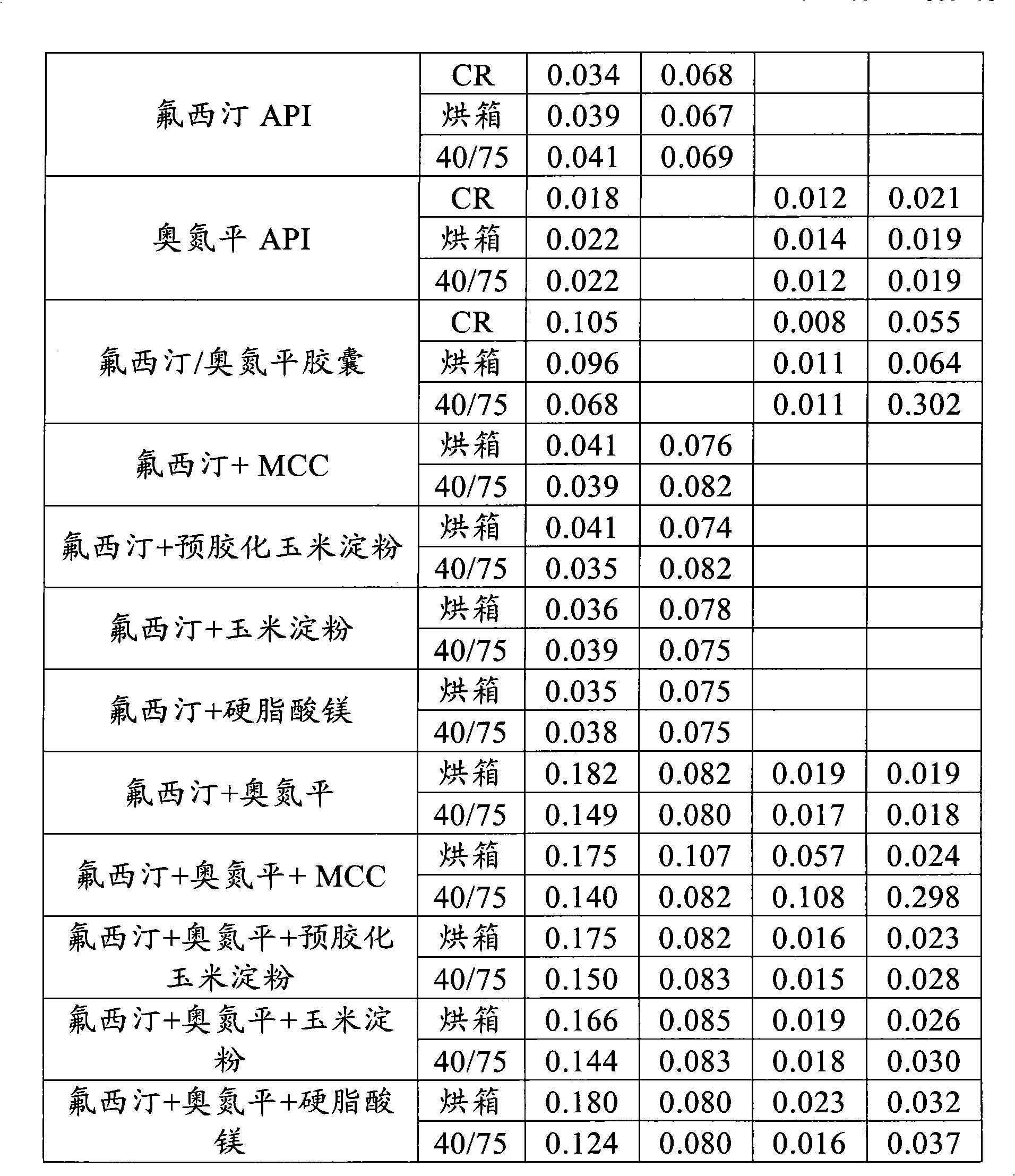
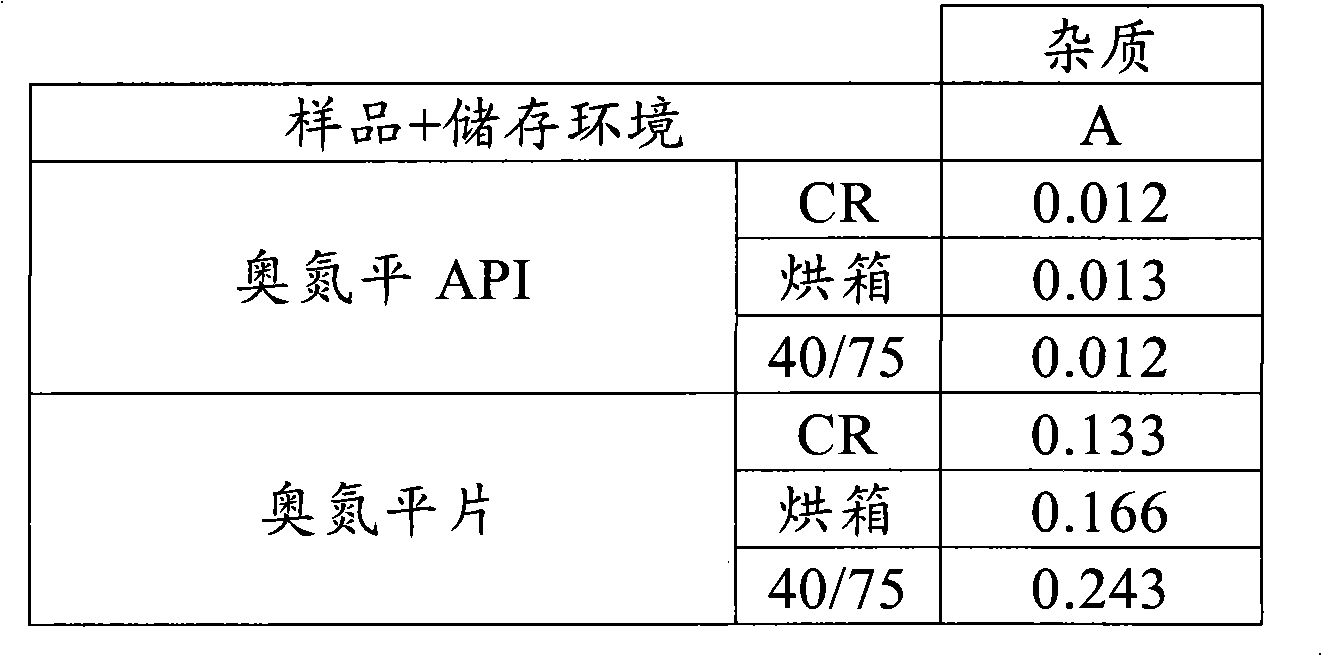
[0100] There was no significant increase in known ...
Embodiment 2
[0102] Composition Weight / unit (mg)
[0103] Part A (Granules)
[0104] Metformin hydrochloride 502.51
[0105] RL / RS 50.00
[0107] water QS
[0108] Magnesium stearate 5.60
[0109] Part B (small piece)
[0110] Pioglitazone hydrochloride 15.00
[0111] Lactose 22.125
[0112] MCC 7.375
[0113] Crospovidone 9.00
[0114] Magnesium stearate 0.50
[0115] Total 620.00
[0116] The ingredients of Part A are suitably wet-granulated, dried, comminuted and blended as is known in the pharmaceutical formulation industry.
[0117] The ingredients of Part B are suitably mixed as is known in the pharmaceutical formulation industry. The resulting granules are compressed into tablets.
[0118] A suitable amount of granules providing a sufficient amount of Part A is filled into a suitable sized capsule, and an appropriate number of Part B tabletlets is added.
Embodiment 3
[0120] Composition Weight / unit (mg)
[0121] Part A (Granules)
[0122] Metformin hydrochloride 502.51
[0123] Povidone K30 20.00
[0124] Microcrystalline cellulose 105.00
[0125] water QS
[0126] Magnesium stearate 2.49
[0127] Part B (small piece)
[0128] Rosiglitazone maleate 5.30
[0129] Lactose 110.20
[0130] Hypromellose E3 4.50
[0131] MCC 16.50
[0132] Sodium starch glycolate 12.00
[0133] water QS
[0134] Magnesium stearate 1.50
[0135] Total 780.00
[0136] The ingredients of Part A are suitably wet-granulated, dried, comminuted and blended as is known in the pharmaceutical formulation industry.
[0137] The ingredients of Part B are suitably wet granulated, dried, comminuted and blended as is well known in the pharmaceutical formulation industry. The resulting granules are compressed into tablets.
[0138] A suitable amount of granules providing a sufficient amount of Part A is filled into a suitable sized capsule, and an appropriate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com